Carbazole compounds, and synthesis and application thereof in OLEDs (Organic Light-Emitting diodes)
A compound, carbazole technology, applied in the field of organic electroluminescent materials, can solve problems such as harsh reaction conditions and increased costs, and achieve the effect of improving device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
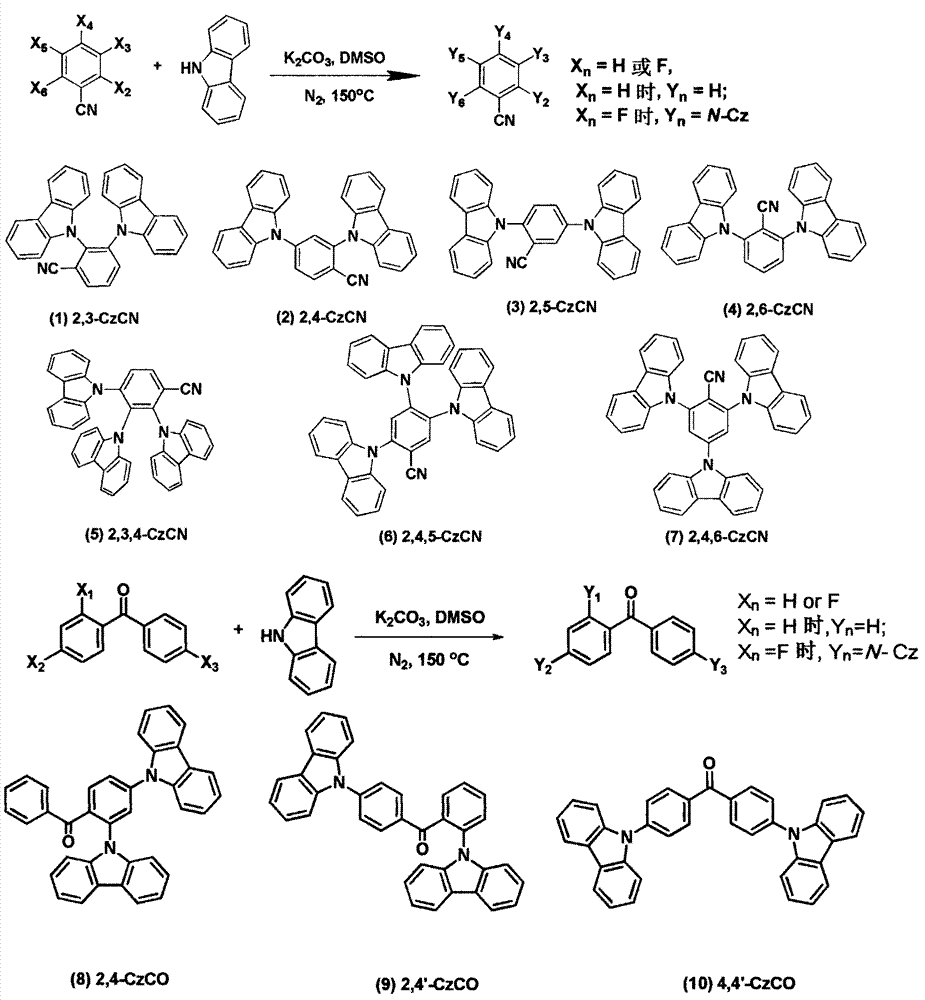
[0013] Embodiment (1): 2, the synthesis of 3-CzCN
[0014] 2,3-Difluorobenzonitrile (0.36g, 2.6mmol), potassium carbonate (2.23g, 16.1mmol), carbazole (0.95g, 5.7mmol), DMSO 8ml, heated to reflux at 150°C for 12h. Cool to room temperature and pour into 200ml of water to precipitate a large amount of solid, stir for 0.5h, filter with suction to obtain a white solid, and purify by column chromatography to obtain a white solid with a yield of 95%. 1 H NMR (300MHz, CDCl 3 ): δppm 8.06 (d, 2H, J=7.5Hz), 7.84 (t, 1H, J=7.8Hz), 7.74 (d, 4H, J=7.2), 7.07-7.01 (m, 12H).
Embodiment (2
[0015] Embodiment (2): 2, the synthesis of 4-CzCN
[0016] 2,4-Difluorobenzonitrile (0.36g, 2.6mmol), potassium carbonate (2.23g, 16.1mmol), carbazole (0.95g, 5.7mmol), DMSO 8ml, heated to reflux at 150°C for 12h. Cool to room temperature and pour into 200ml of water to precipitate a large amount of solid, stir for 0.5h, filter with suction to obtain a white solid, and purify by column chromatography to obtain a white solid with a yield of 84%. 1 H NMR (300MHz, CDCl 3 ): δppm 8.19-8.13(m, 5H), 7.92(d, 2H, J=5.4Hz), 7.57(d, 2H, J=5.1Hz), 7.50-7.44(m, 4H), 7.38-7.33(m , 6H).
Embodiment (3
[0017] Embodiment (3): 2, the synthesis of 5-CzCN
[0018] 2,5-difluorobenzonitrile (0.36g, 2.6mmol), potassium carbonate (2.23g, 16.1mmol), carbazole (0.95g, 5.7mmol), DMSO 8ml, heated at reflux at 150°C for 12h. Cool to room temperature and pour into 200ml of water to precipitate a large amount of solid, stir for 0.5h, filter with suction to obtain a white solid, and purify by column chromatography to obtain a white solid with a yield of 92%; 1 H NMR (300MHz, CDCl 3 ): δppm 8.20 (t, 5H, J = 5.7Hz), 8.10-8.06 (m, 1H), 7.87 (d, 1H, J = 8.7Hz), 7.58-7.36 (m, 12H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com