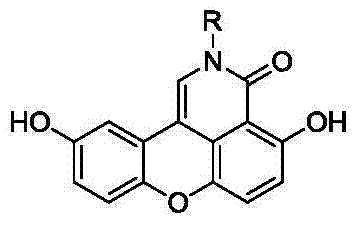
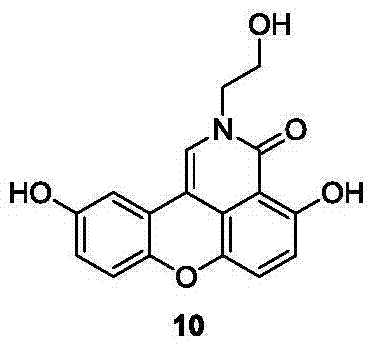
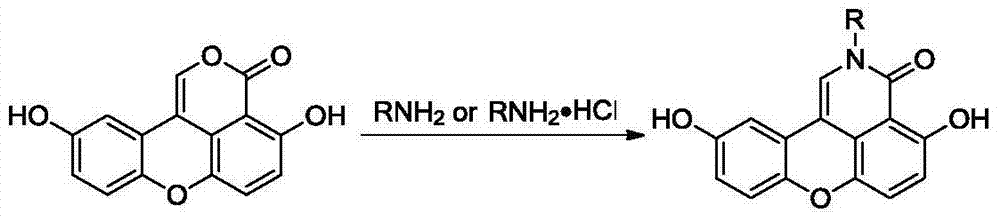Sparganiaceae lactone B derivative as well as preparation method and use thereof
A technology of black triangular lactone and derivatives, which is applied in the fields of chemical and pharmaceutical invention, can solve the problems of difficulty in extraction and separation, source limitation, low natural content of black triangular lactone B, etc., and achieves simple preparation method, easy operation, The effect of high tumor cell growth inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Add 50 mg of black trigonalide B to methanol solution of ammonia gas (self-made, about 10 mol / L, 2 mL), reflux for 8 h, evaporate the solvent to dryness under reduced pressure, and separate by column chromatography (eluent is petroleum ether / ethyl acetate Ester = 3 / 1) to obtain 141 mg of the compound as a yellow solid, with a yield of 82%. 1 H NMR(400MHz,DMSO)δ12.32(s,1H),11.72(s,1H),9.37(s,1H),7.40(s,1H),7.21(d,J=8.7Hz,1H),7.11 (s,1H),6.91(d,J=8.7Hz,1H),6.81(d,J=8.2Hz,1H),6.71(d,J=8.6Hz,1H); 13 C NMR (400MHz, DMSO) δ163.3, 157.1, 152.7, 143.4, 141.2, 126.8, 123.9, 122.2, 119.7, 118.9, 118.3, 116.3, 108.8, 108.3, 106.6; HRMS: C 15 h 9 o 4 N[M+Na] + The theoretical value of m / z is 290.0424, and the measured value is 290.0431.
Embodiment 2
[0025]
[0026] Add 50 mg of black trigonalide B to an ethanol solution of methylamine (31-35%, 2 mL), and follow the method of Example 1 to obtain 245 mg of compound, a yellow solid, with a yield of 86%. 1 H NMR(400MHz,DMSO)δ12.29(s,1H),9.42(s,1H),7.74(s,1H),7.17(d,J=8.7Hz,1H),7.08(d,J=2.2Hz ,1H),6.91(d,J=8.8Hz,1H),6.80(d,J=8.7Hz,1H),6.72(dd,J=8.8,2.2Hz,1H),3.52(s,3H); 13 C NMR (400MHz, DMSO) δ171.5, 159.6, 147.7, 145.6, 143.7, 135.7, 128.4, 125.1, 121.2, 119.2, 119.4, 117.0, 113.8, 108.6, 105.6, 36.9; HRMS: C 16 h 11 o 4 N[M+Na] + The theoretical value of m / z is 304.0580, and the measured value is 304.0589.
Embodiment 3
[0028]
[0029] Add n-butylamine (21 mg) to the isopropanol solution of black trilendolide B (50 mg), and follow the method of Example 1 to obtain 347 mg of the compound as a light yellow solid with a yield of 78%. 1 H NMR(400MHz,DMSO)δ12.36(s,1H),9.38(s,1H),7.78(s,1H),7.21(d,J=8.7Hz,1H),7.16(d,J=2.4Hz ,1H),6.92(d,J=8.8Hz,1H),6.82(d,J=8.8Hz,1H),6.73(dd,J=8.8,2.6Hz,1H),3.97(t,J=7.3Hz ,2H),1.77–1.67(m,2H),1.35(dd,J=14.7,7.7Hz,2H),0.94(t,J=7.4Hz,3H); 13 C NMR (400MHz, DMSO) δ170.1, 158.9, 150.6, 144.2, 140.1, 127.8, 126.4, 123.3, 119.8, 118.0, 117.5, 117.0, 111.5, 110.3, 106.2, 45.4, 30.2, 20.7, 14.2; HRMS: C 19 h 17 NO 4 [M+Na] + The theoretical value of m / z is 346.1050, and the measured value is 346.1055.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com