Vaccine for preventing swine transmissible gastroenteritis
An infectious and gastroenteritis technology, applied in the field of genetic engineering, can solve problems such as poor immunogenicity, and achieve the effects of excellent clinical application prospects, good safety and strong protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1 Construction of recombinant bacteria of the present invention
[0068] 1. Preparation method
[0069] 1 Construction of double-promoter eukaryotic plasmid
[0070] 1.1 RT-PCR amplification of S gene and N gene
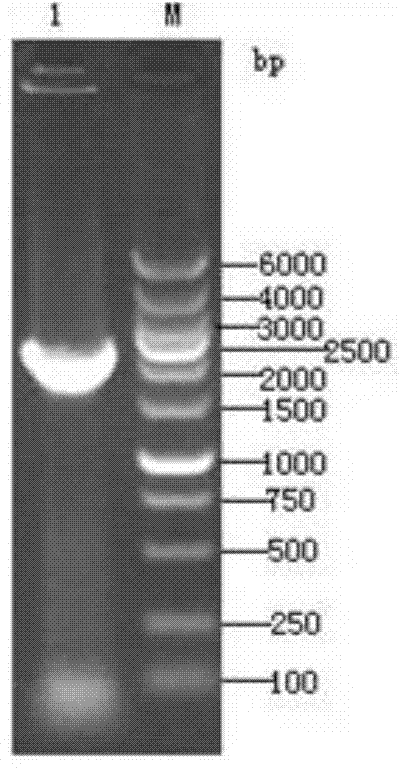
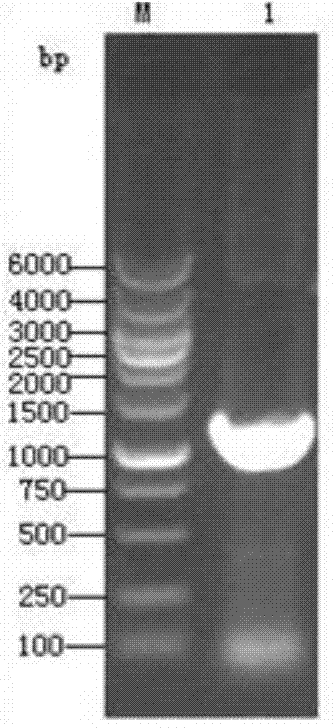
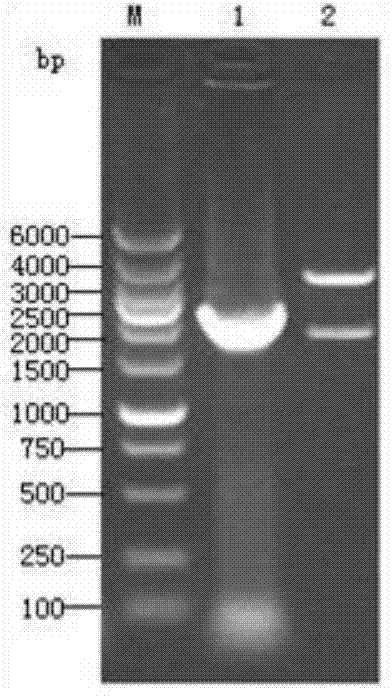
[0071] According to the S gene sequence of TGEV, a pair of primers were designed to amplify the 5’-1kb antigenic region of the S gene (named Sa, including four antigenic sites A, B, C, and D), and the upstream primer S1: 5’-CG GCTAGC ACCATGGAAAAACTATTTG-3', introduce restriction site NheI; downstream primer S2: 5'-CC AAGCTT TTAGCCACTAAGTAGCGTC-3', introduce restriction site HindIII. Design a pair of primers according to the N gene sequence, the expected amplification length is, upstream primer N1: 5'-CG GAATTC AAAATGGCCAACCAGGGAC-3', introduce restriction site EcoRI; downstream primer N2: 5'-CC CTCGAG AAGACGAGCATCTC-3', the restriction site XhoI was introduced.
[0072] Take TGEV cell culture virus liquid, extract viral RNA according to th...
experiment example 1
[0130] Experimental example 1 Characteristic identification and protective detection of recombinant attenuated Salmonella of the present invention
[0131] 1. Experimental method
[0132] Get the recombinant Salmonella SL7207 (pVAX-S) prepared in Example 1 499-650 ), according to the following method for identification:
[0133] 1 Identification of biological characteristics of strain SL7207 (pVAXD-N-Sa)
[0134] 1.1 Microscopic examination of Gram staining of bacterial strain SL7207 (pVAXD-N-Sa) and control strain
[0135]SL7207(pVAXD-N-Sa) and control strains SL7207(pVAX-S), SL7207(pVAX-N), SL7207(pVAX-S-N) and SL7207(pVAXD) were respectively streaked on LB plates containing 100 μg / mL Kan, Cultivate for 16 hours, pick a single colony, inoculate 5 mL of LB liquid medium containing 100 μg / mL Kan, culture at 37 °C, shake at 300 r / min until the OD600 is about 0.6, take the bacterial liquid for Gram staining and microscopic examination, observe and record the bacteria form. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com