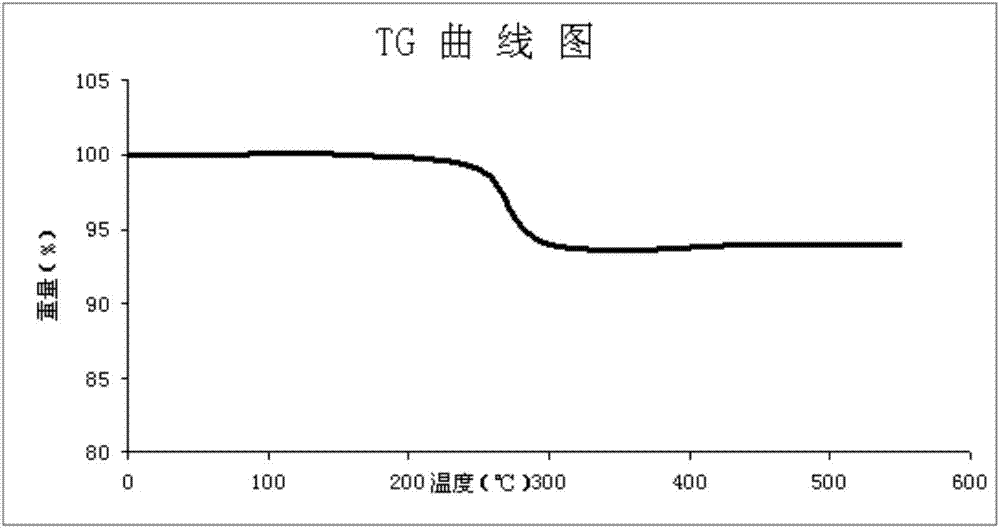
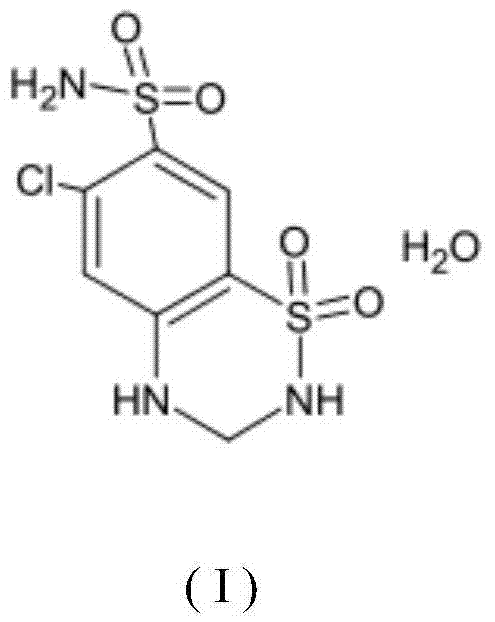
Stable hydrochlorothiazide crystalline compound, and composite enalapril maleate pharmaceutical composition thereof
A technology of enalapril maleate and crystal compounds, which is applied in the field of medicine, can solve the problems of slow absorption rate and low solubility, and achieve the effects of small fluctuations, good solubility, and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] First dissolve the hydrochlorothiazide solid in the sodium hydroxide acetone solution, adjust the temperature of the solution to drop a mixed solvent of chloroform and absolute ethanol under the condition of 50° C., the volume ratio of the chloroform and absolute ethanol is 1: 1.5, the feeding speed of the chloroform and dehydrated ethanol is 2ml / min, and the stirring speed is 20 rpm when the described feeding of chloroform and dehydrated ethanol, after the mixed solvent is added dropwise, Continue heat preservation and stirring for 30 minutes, lower the temperature to 30° C., heat preservation and stirring for 30 minutes to obtain crystals: filter, wash the filter cake with chloroform, and vacuum dry for 2-4 hours to obtain hydrochlorothiazide crystal compound.
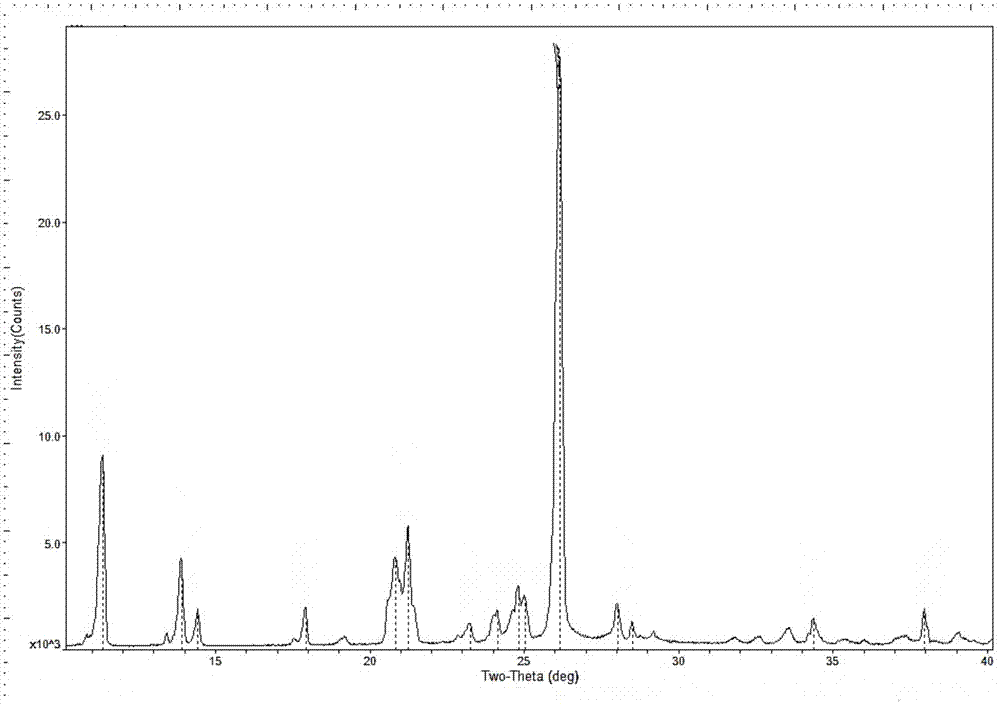
[0084] X-ray powder diffraction measured by using Cu-Kα rays at 2θ of 11.4°, 13.9°, 14.4°, 17.9°, 20.8°, 21.2°, 24.1°, 24.8°, 25.0°, 26.2°, 28.0°, 38.0 ° shows a characteristic peak, and its X-ray powder diffr...
Embodiment 2
[0086] First dissolve the hydrochlorothiazide solid in the sodium hydroxide acetone solution, adjust the temperature of the solution to drop a mixed solvent of chloroform and absolute ethanol under the condition of 50° C., the volume ratio of the chloroform and absolute ethanol is 1: 1.25, the feeding speed of the chloroform and dehydrated ethanol is 3ml / min, and the stirring speed is 30 rpm when the described chloroform and dehydrated ethanol is added, after the mixed solvent is added dropwise, Continue heat preservation and stirring for 30 minutes, lower the temperature to 30° C., heat preservation and stirring for 30 minutes to obtain crystals: filter, wash the filter cake with chloroform, and vacuum dry for 2-4 hours to obtain hydrochlorothiazide crystal compound.
[0087] X-ray powder diffraction measured by using Cu-Kα rays at 2θ of 11.4°, 13.9°, 14.4°, 17.9°, 20.8°, 21.2°, 24.1°, 24.8°, 25.0°, 26.2°, 28.0°, 38.0 ° shows a characteristic peak, and its X-ray powder diffra...
Embodiment 3
[0089]First dissolve the hydrochlorothiazide solid in the sodium hydroxide acetone solution, adjust the temperature of the solution to drop a mixed solvent of chloroform and absolute ethanol under the condition of 51° C., the volume ratio of the chloroform and absolute ethanol is 1: 1.1, the feeding speed of the chloroform and dehydrated ethanol is 5ml / min, and the stirring speed is 45 rpm when the described feeding of chloroform and dehydrated ethanol, after the mixed solvent is added dropwise, Continue to insulate and stir for 30 minutes, lower the temperature to 31° C., and insulate and stir for 30 minutes to obtain crystals: filter, wash the filter cake with chloroform, and vacuum-dry for 2-4 hours to obtain hydrochlorothiazide crystalline compound.
[0090] X-ray powder diffraction measured by using Cu-Kα rays at 2θ of 11.4°, 13.9°, 14.4°, 17.9°, 20.8°, 21.2°, 24.1°, 24.8°, 25.0°, 26.2°, 28.0°, 38.0 ° shows a characteristic peak, and its X-ray powder diffraction pattern i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com