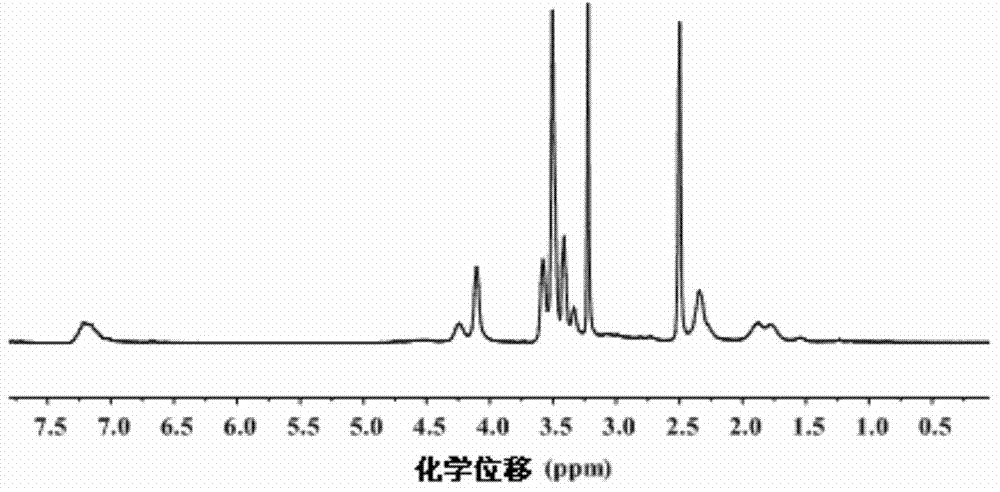
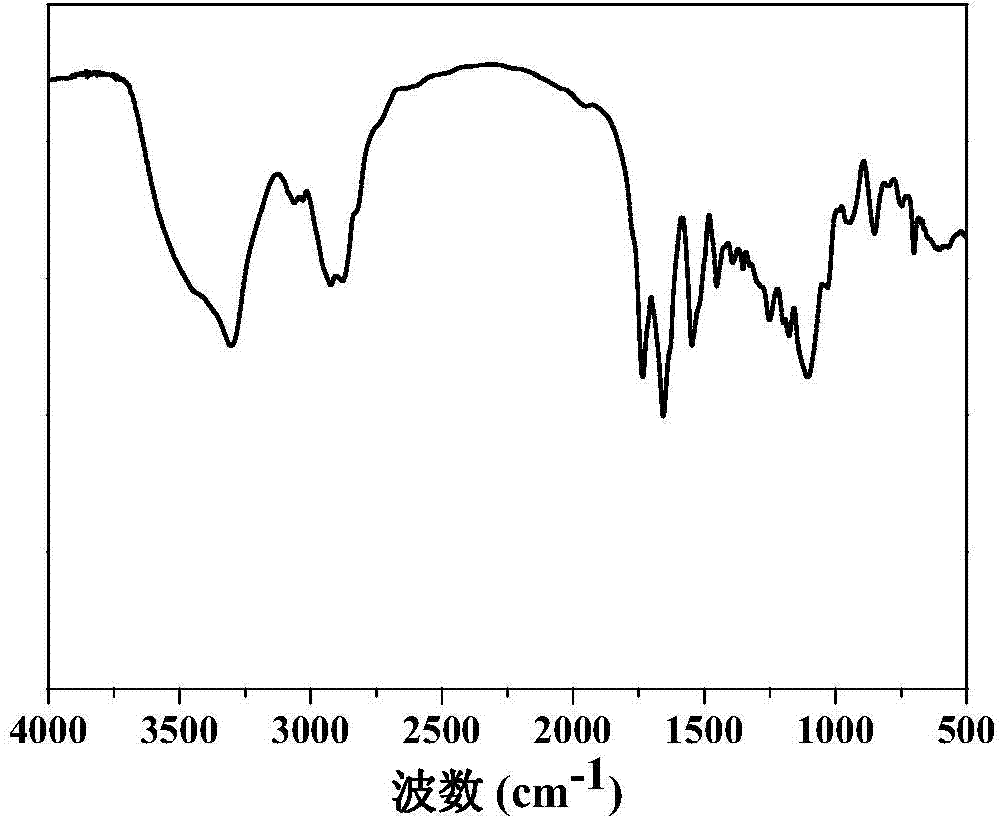
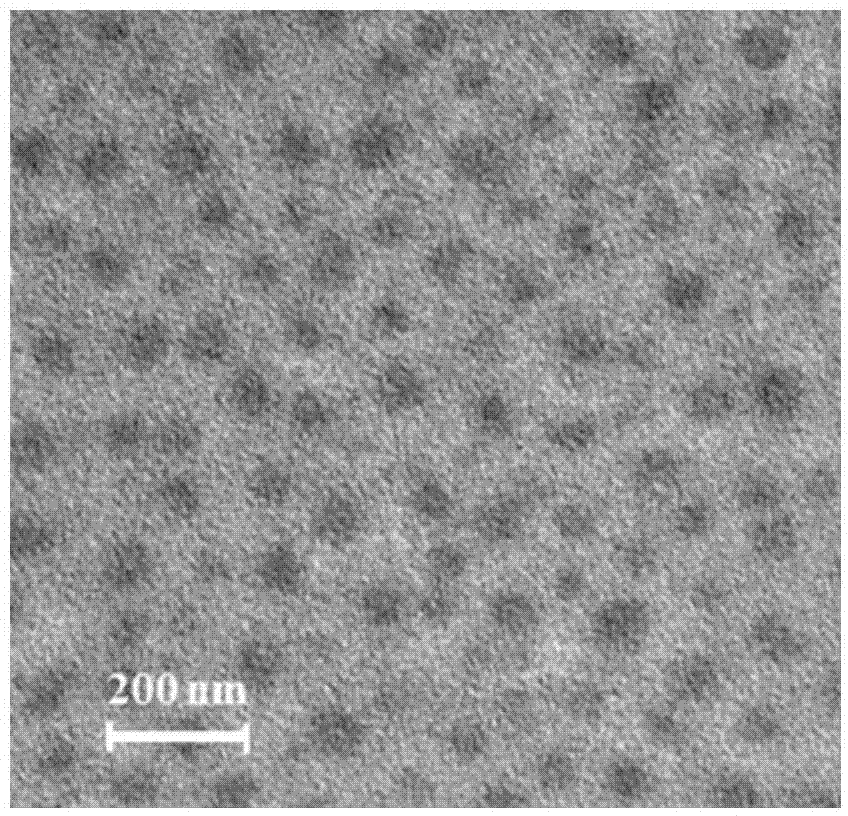
Nanogel, preparation method of nanogel and anti-tumor nanogel drug loading system and preparation method of anti-tumor nanogel drug loading system
A technology of nanogel and summation, which is applied in antitumor drugs, liquid delivery, drug combination, etc., can solve the problems of poor water solubility and stability, and large drug side effects, and achieve good biocompatibility, tumor inhibition, and promotion of Effects of tumor targeting and endocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The invention provides a kind of preparation method of nanogel, comprising the following steps:
[0081] In the presence of a phenylboronic acid initiator and a morpholine initiator, reacting L-glutamic acid oligoethylene glycol monomethyl ether ester-N-cyclic acid anhydride in an organic solvent to obtain a reaction solution;
[0082] Mixing the reaction solution with L-cystine-N-cyclic anhydride and L-phenylalanine-N-cyclic anhydride to react to obtain a nanogel;
[0083] The nanogel includes phenylboronic acid group, morpholine group, glutamic acid oligoethylene glycol monomethyl ether ester chain link, phenylalanine chain link and cystine link;
[0084] The nano gel forms a cross-linked network structure through disulfide bonds; the nano gel is a polyamino acid material.
[0085] The present invention aims to solve the problem of intelligent delivery of anti-tumor drugs. In order to overcome the shortcomings of low utilization rate and large side effects of anti-tu...
Embodiment 1
[0119] Weigh 10 g of L-glutamic acid and 20 mL of ethylene glycol monomethyl ether and mix them under stirring conditions, add 5 mL of concentrated sulfuric acid dropwise, and react under stirring conditions to obtain a reaction solution. Adjusting the pH value of the reaction solution to neutral, performing centrifugation, and then dissolving the centrifuged solid with methanol to obtain a mixed solution. Pour the mixed solution into isopropanol for centrifugation, and vacuum-dry the solid obtained by centrifugation to obtain L-glutamic acid oligoethylene glycol monomethyl ether ester.
Embodiment 2~5
[0121] According to the method of Example 1, L-glutamic acid oligoethylene glycol monomethyl ether esters were prepared respectively; the difference is that the raw materials for reacting with 10g of L-glutamic acid were: Methyl ether, 60mL triethylene glycol monomethyl ether, 80mL tetraethylene glycol monomethyl ether and 100mL pentaethylene glycol monomethyl ether.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com