Crystal form of sorafenib tosylate, and preparation method thereof
A technology based on p-toluenesulfonic acid and fennel, which is applied in the field of medicine, can solve the problems of low crystallinity and difficulty in meeting the production requirements of industrialized large-scale industries, and achieve good fluidity, cheap solvents, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of Sorafenib p-Toluenesulfonate Type B Crystal
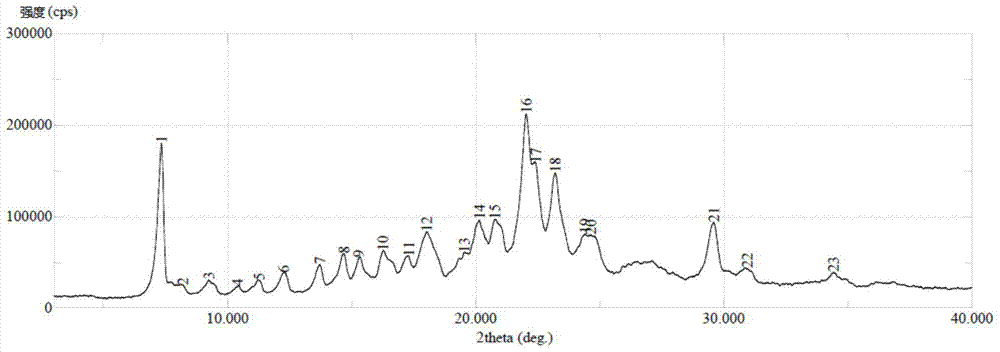
[0037] Take 10.0g of sorafenib, add 200mL of tetrahydrofuran, stir to dissolve, add 2g of activated carbon, stir for 30min, filter, cool the filtrate to -15°C, add dropwise a solution of 4.3g of p-toluenesulfonic acid monohydrate dissolved in 13mL of tetrahydrofuran, After the addition, stir and crystallize for 1 hour, filter, and rinse the filter cake with 100ml isopropyl ether to obtain crystals, which have figure 1 X-ray powder diffraction pattern shown.
Embodiment 2
[0038] Example 2 Preparation of Sorafenib p-Toluenesulfonate Type C Crystal
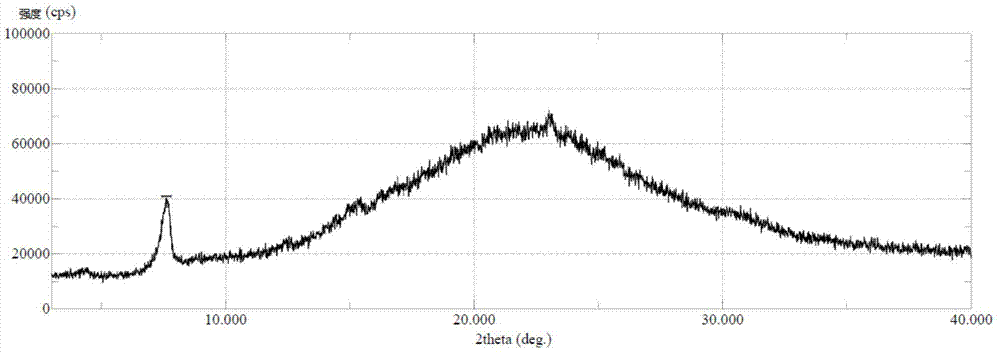
[0039] The crystals prepared in Example 1 were dried under reduced pressure at 60° C. for 5 hours to obtain 12.9 g of type C crystals of sorafenib p-toluenesulfonate with a purity of 99.9%. it has figure 2 X-ray powder diffraction pattern shown.
Embodiment 3
[0040] Example 3 Preparation of Amorphous Sorafenib p-toluenesulfonate
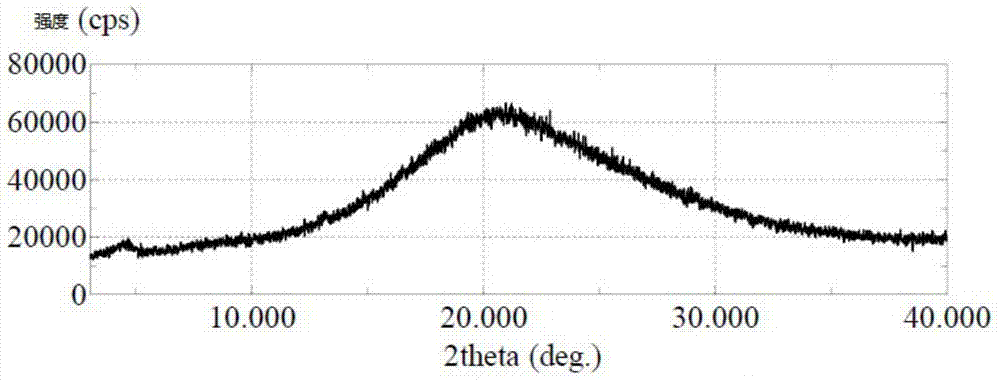
[0041] The product of Example 1 or Example 2 was dried under reduced pressure at 80° C. for 20 hours to obtain amorphous sorafenib p-toluenesulfonate with a purity of 99.87%. it has image 3 X-ray powder diffraction pattern shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com