Lyase of self-cleaving escherichia coli, and applications thereof
A technology of Escherichia coli and lyase, applied in the field of microorganisms, can solve the problems of refractory host bacteria, inability to realize controllable self-cleavage of Escherichia coli, and conservation of lyase action sites, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the construction of the lyase that can induce Escherichia coli self-cleavage
[0022] (1) Synthesized in Nanjing GenScript Biotechnology Co., Ltd., which can express the cleavage enzyme ClyN ClyN genetic DNA sequence. The synthetic sequence was loaded into the pUC57 plasmid. by ClyN The gene is used as a template, and NcoI and XhoI restriction sites are added to the two ends of the target gene respectively. The primers are designed as follows:
[0023] Forward primer: 5-ATAT CCATGG GCATGCCTCCATCATTACG-3 (SEQ.ID.NO.3)
[0024] NCOI
[0025] Reverse primer: 5-TATA CTCGAG GTAGTTCAGGGTCTGGCCTG-3 (SEQ.ID.NO.4)
[0026] wxya
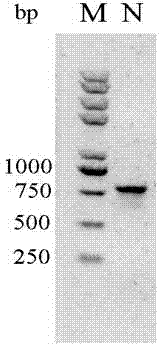
[0027] Using 2 μl of the gene as a template, add 1 μg of primers for PCR amplification. The PCR amplification procedure is as follows: 1) 94 °C, 5 min; 2) 94 °C, 30 sec, 62 °C, 45 sec, 72 °C, 45 sec , 30 cycles; 3) 72 ℃, 10 min; the product was recovered by gel electrophoresis, and the electrophoresis pattern was as follows: f...
Embodiment 2
[0029] Example 2, Verification of ClyN-induced Escherichia coli self-lysis
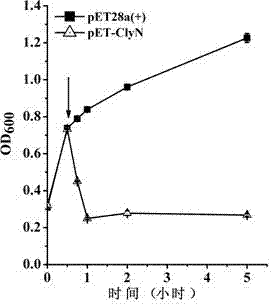
[0030] Culture Escherichia coli BL21(DE3) / pET-ClyN to OD 600 =0.8, after adding IPTG to 0.5 mM, use a microplate reader to monitor the change of the absorbance value of the bacterial solution at 600 nm. At the same time, the bacterial solution without IPTG was used as a negative control. The final pyrolysis curve obtained is shown in the appendix figure 2 . The results showed that ClyN could induce rapid self-lysis of the host strain resulting in a rapid decrease in absorbance at 600 nm.
Embodiment 3
[0031] Example 3, Verification of the results of changes in ATP in the culture medium after ClyN-induced Escherichia coli self-lysis
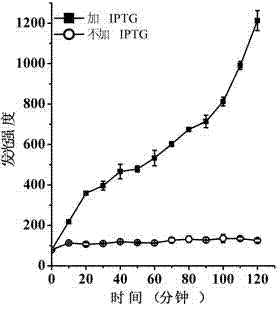
[0032] Culture Escherichia coli BL21(DE3) / pET-ClyN to OD 600 =0.8, after adding IPTG to 0.5 mM, use the luciferase detection kit to detect the change of the luminescence value in the bacterial solution. At the same time, the bacterial solution without IPTG was used as a negative control. The luminescence curve obtained from the test is shown in the appendix image 3 . The results showed that a large amount of ATP could be detected in the culture medium after ClyN induced the self-lysis of the host Escherichia coli.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com