Cell line method pyrogen detection method and kit
A cell line and cell technology, applied in the field of medicine, can solve the problems of poor dose dependence, low sensitivity, and difficult to obtain cells, and achieve the effect of simple operation, high sensitivity, and good dose dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 The method for detecting pyrogens based on the cell line method of HL-60 cells
[0040] 1.1 Materials and reagents
[0041] Cell culture medium: 400 mL of IMDM culture medium (purchased from Gibco), add 100 mL of fetal bovine serum (purchased from Gibco) and mix well.
[0042] Cells: HL-60 were purchased from the Institute of Biochemistry and Cells, Academy of Life Sciences, Chinese Academy of Sciences; passage: F10.
[0043] ELISA kit: human interleukin-6 (IL-6) enzyme-linked immunosorbent (ELISA) kit (purchased from BD OptEIA TM )
[0044] Consumables: cell counting plate, sterile plastic centrifuge tube (1.5mL, 50mL), disposable plastic pipette (sterile, single package, 10mL), 25cm 2 Culture flasks, 24-well or 96-well cell culture plates.
[0045] Diluent: IMDM culture medium (purchased from Gibco) 490mL, add fetal bovine serum (purchased from Gibco) 10mL and mix well.
[0046] 1.2 Instruments
Embodiment 2
[0071] The method for the detection pyrogen of embodiment 2 different cell densities
[0072] The cell suspension prepared in Example 1 was diluted to the following concentrations respectively (i.e. the cell density was ): 1×10 5 pcs / mL, 5×10 5 pcs / mL, 1×10 6 pcs / mL, 5×10 6 pcs / mL, 1×10 7 / mL and 5×10 7 individual / mL. Add 400 μl / well to 24-well culture plate respectively.
[0073] Add the 0.5Eu / mL LPS standard solution described in Example 1 into a 24-well cell culture plate at 100 μL / well for pyrogen stimulation. 2 , 37 ° C incubator to continue culturing for 2 days, the percentage is the volume percentage of fetal bovine serum in the IMDM culture medium. The cell supernatant was taken, and the amount of IL-6 was determined according to the instructions of the IL-6 ELISA kit. The results are shown in Table 1.
[0074] Table 1 Detection results of different cell densities
[0075] Cell density (unit / mL)
[0076] The results in Table 1 show that after the pyr...
Embodiment 3
[0077] The method for the detection pyrogen of embodiment 3 different cultivation time
[0078] The cell suspension prepared in Example 1 was adjusted to a cell concentration of 5 × 10 6 cells / mL, added to 24-well culture plate at 400 μl / well.
[0079] Add the 0.5Eu / mL LTA standard solution described in Example 1 into a 24-well cell culture plate at 100 μL / well for pyrogen stimulation. 2 , 37 ° C incubator to continue culturing for 2 days, the percentage is the volume percentage of fetal bovine serum in the IMDM culture medium. The cell supernatant was taken, and the amount of IL-6 was determined according to the instructions of the IL-6 ELISA kit, and the results are shown in Table 2.
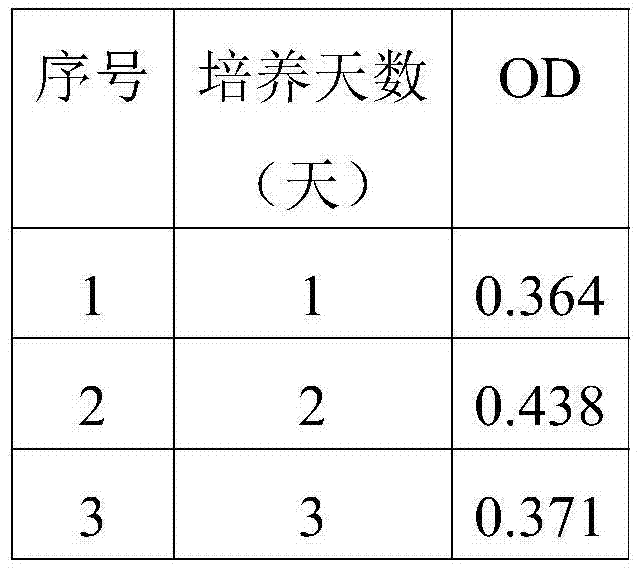
[0080] Table 2 Test results at different times
[0081]
[0082] The results in Table 2 show that the secretion of IL-6 is the highest after 2 days of pyrogen-stimulated cells, so it is determined that 2 days of pyrogen-treated cells is the best action time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com