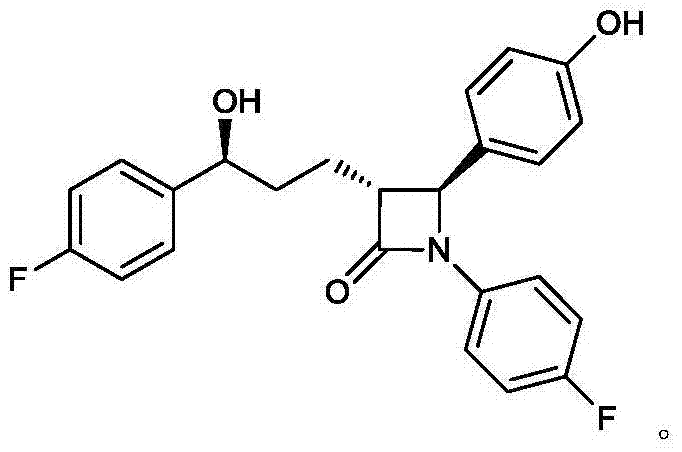
Method for separating and detecting enantiomer of ezetimibe key intermediate
An enantiomer and ezetimibe technology, which is applied in the field of drug analysis, can solve problems such as the analysis and detection method of key intermediates of ezetimibe, and achieve safety and effectiveness, high sensitivity, and method accurate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of Derivatized Product of Ezetimibe Key Intermediate A
[0037] At room temperature, add 0.1mL of isopropanol and 10mg of ezetimibe key intermediate A to a 1.5ml EP tube, shake, then add 35μl of 1mol / L sulfuric acid solution, place on a shaker for 2h, then add Dilute the sample with 0.5ml of isopropanol and transfer it to the filter, filter, wash the EP tube and filter with a small amount of isopropanol, take the filter cake, evaporate the solvent at room temperature, and obtain the derivative of the key intermediate A of ezetimibe chemical products.
Embodiment 2
[0038] Example 2: Preparation of Enantiomeric Derivatized Products of Ezetimibe Key Intermediate A
[0039] At room temperature, add 0.1 mL of isopropanol and 10 mg of the enantiomer of ezetimibe key intermediate A to a 1.5 ml EP tube, shake, then add 35 μl of 1 mol / L sulfuric acid solution, place on a shaker Shake it up for 2 hours, then add 0.5ml of isopropanol to dilute the sample and transfer it to the filter, filter, wash the EP tube and filter with a small amount of isopropanol, take the filter cake, and evaporate the solvent at room temperature to obtain ezetimibe Derivatization products of enantiomers of key intermediate A.
Embodiment 3
[0041] Instruments and Conditions:
[0042] High performance liquid chromatography: Agilent, 1260Bin Pump, 1260VWD detector, 1260TCC column thermostat, 1260ALS autosampler, ChemStation workstation;
[0043] Column: IC (150mm×4.6mm×5μm);
[0044] Mobile phase: water: acetonitrile: tetrahydrofuran = 65: 31.6: 3.4;
[0045] Flow rate: 1.0ml / min;
[0046] Detection wavelength: 230nm;
[0047] Column temperature: 30°C;
[0048] Injection volume: 20 μl.
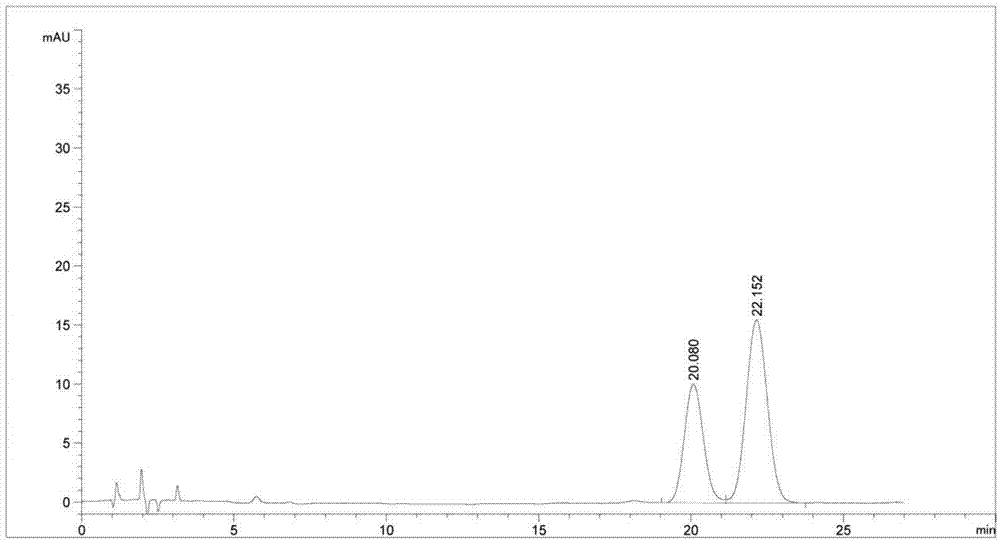
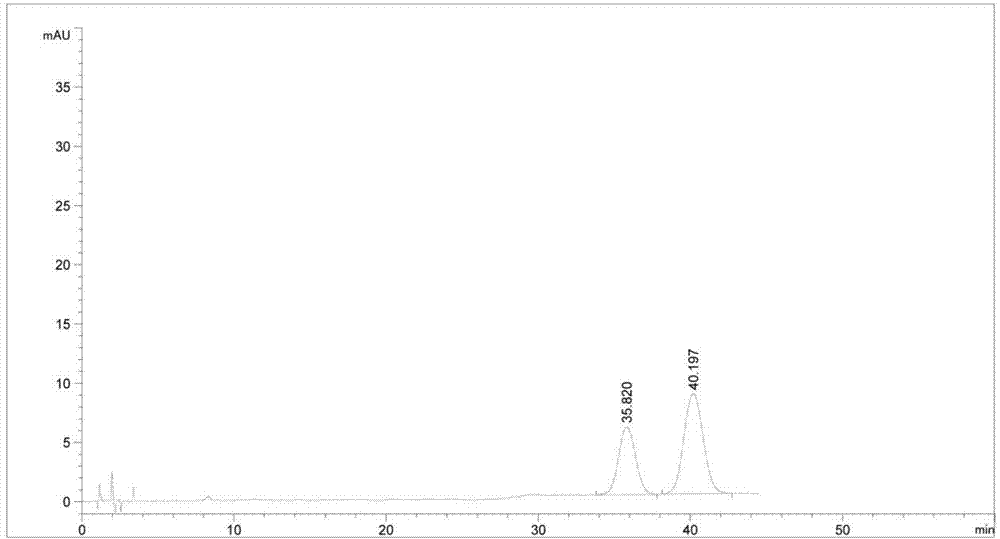
[0049] Experimental procedure: take the derivatized products in Examples 2 and 3 in equal amounts, mix them, dissolve and dilute them with acetonitrile-water mixed solvent (acetonitrile:water=40:60) to a concentration of about 1 mg / ml each, and measure them precisely Inject 20 μl into the liquid chromatograph, record the chromatogram, and see the results in figure 1 .
[0050] figure 1 The one with a retention time of 22.152 min is the derivatization product of the key intermediate A of ezetimibe, and the one with a reten...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com