Ferrocene high-nitrogen energetic eutectic
A high-nitrogen-containing, eutectic technology, applied in the preparation of metallocenes, aromatic nitration compositions, organic compounds, etc., can solve the problems of easy migration and volatility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
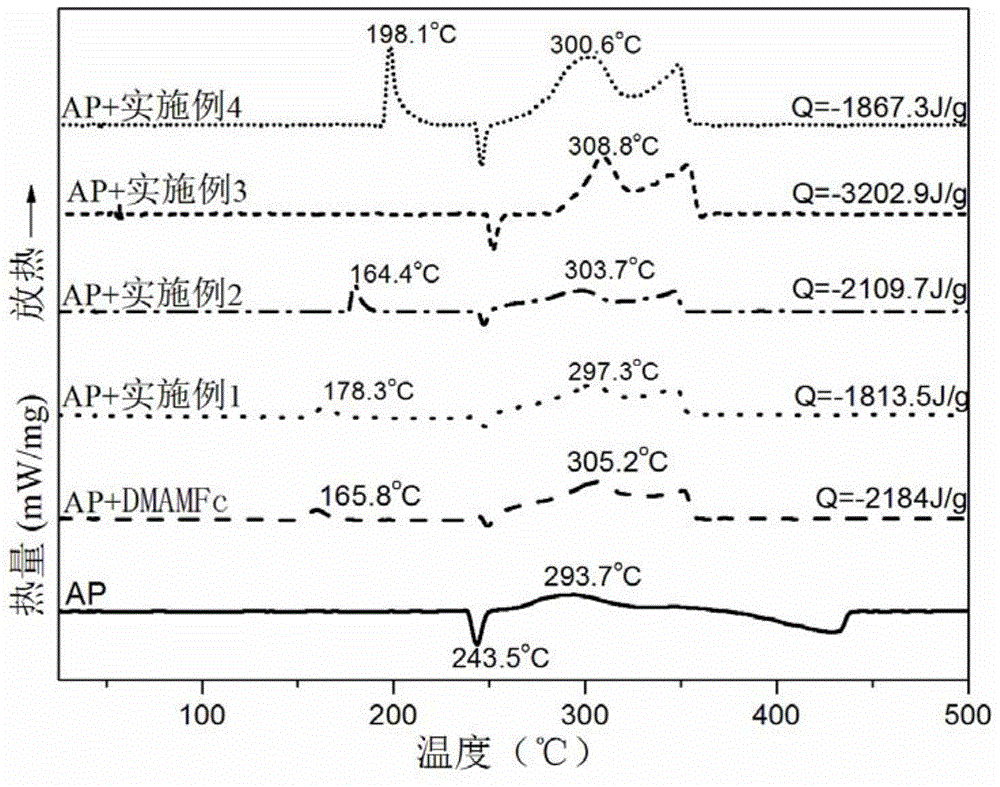
Examples
Embodiment 1
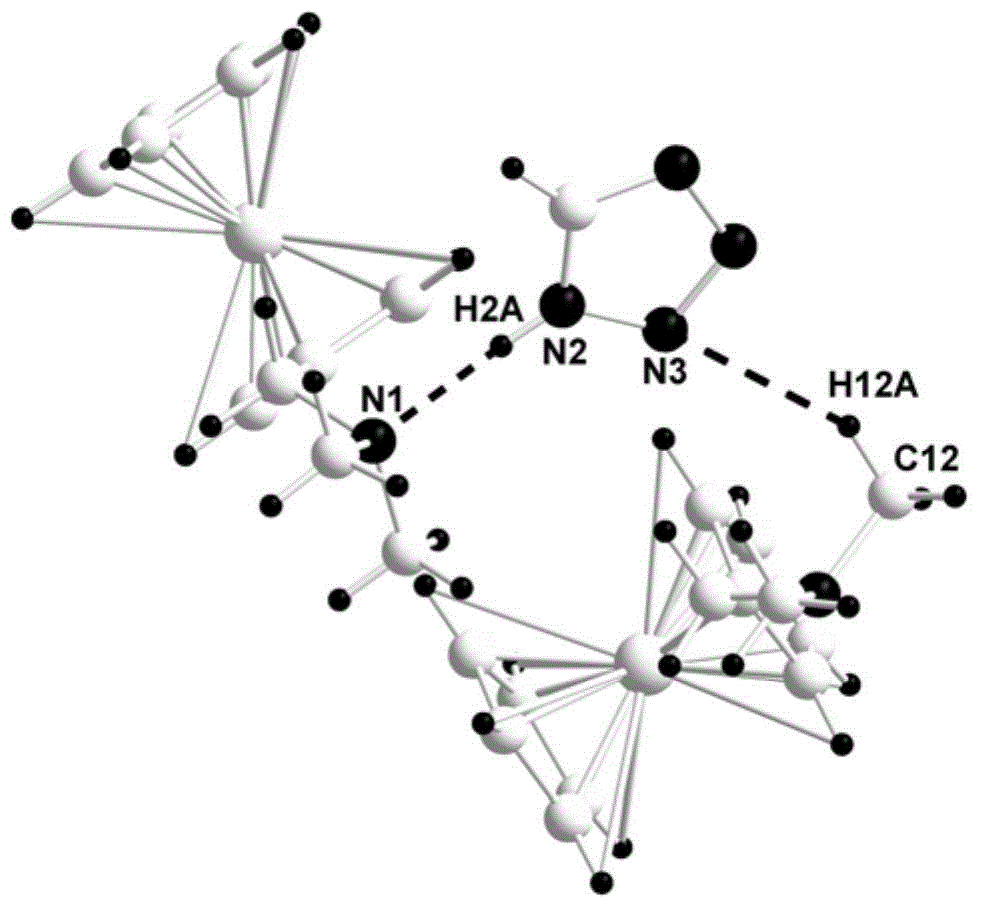
[0018] Taking the preparation of N,N-dimethylaminomethyl ferrocene and tetrazole co-crystal as an example, the reaction equation and preparation method are as follows:
[0019]
[0020] Add 0.070g (1mmol) of tetrazole into a round-bottomed flask containing 15mL of acetone, and stir until the solution is completely transparent; dissolve 0.243g (1mmol) of N,N-dimethylaminomethylferrocene in 15mL of acetone, Then it was slowly added dropwise to a round-bottomed flask with a constant pressure funnel, an orange precipitate was formed immediately, filtered with suction, the filter cake was washed 3 times with acetone, and then placed in a vacuum drying oven at 70°C for 24 hours to obtain N, N-dimethylaminomethyl ferrocene and tetrazolium eutectic powder, its structural characterization data are as follows:
[0021] FT-IR (KBr, cm -1 ): 3300, 2649, 1473, 1025; 1 H NMR (600MHz, DMSO) δ: 8.55(s, 1H), 4.31(s, 2H), 4.23(s, 2H), 4.19(s, 5H), 3.77(s, 2H), 2.42(s, 6H) ; 13 C NMR (600...
Embodiment 2
[0024] Taking the preparation of N,N-dimethylaminomethyl ferrocene and picric acid cocrystal as an example, the reaction equation and preparation method are as follows:
[0025]
[0026] Add 0.229g (1mmol) of picric acid into a round-bottomed flask containing 15mL of methanol, and stir until the solution is completely transparent; dissolve 0.243g (1mmol) of N,N-dimethylaminomethylferrocene in 15mL of methanol, and then Slowly drop it into a round-bottomed flask with a constant pressure funnel, a red precipitate formed immediately, filter it with suction, wash the filter cake with methanol three times, and then dry it in a vacuum oven at 70°C for 24 hours to obtain N,N- Dimethylaminomethyl ferrocene and picric acid eutectic powder, its structural characterization data are as follows:
[0027] FR-IR (KBr, cm -1 ): 3021, 1551, 1326; 1 H NMR (600MHz, DMSO) δ: 8.59(s, 2H), 4.41(s, 2H), 4.32(s, 2H), 4.23(s, 5H), 4.10(d, J=4.2Hz, 2H), 2.65 (s, 6H); 13C NMR (600 MHz, DMSO) δ: 1...
Embodiment 3
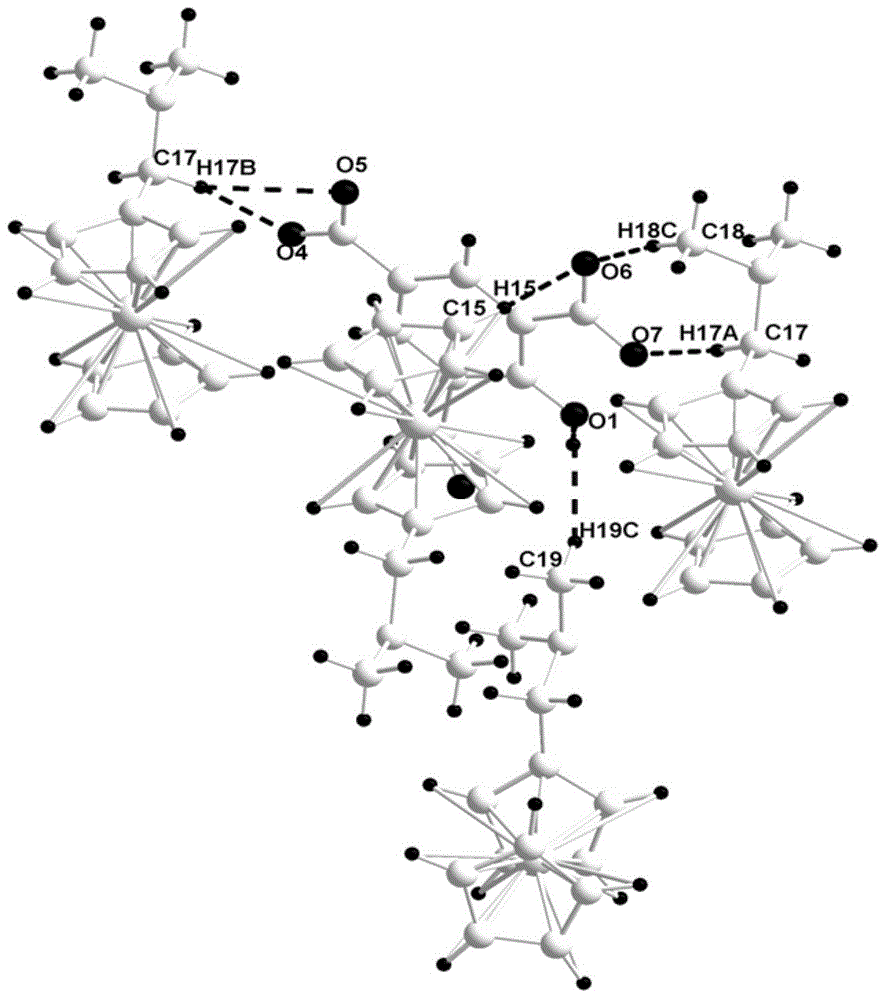
[0030] Taking the preparation of N,N-dimethylaminomethylferrocene and trinitrotoluene cocrystal as an example, the reaction equation and preparation method are as follows:
[0031]
[0032] Add 0.227g (1mmol) trinitrotoluene into a round bottom flask filled with 15mL chloroform, and stir until the solution is completely transparent; dissolve 0.243g (1mmol) N,N-dimethylaminomethylferrocene in 15mL chloroform , and then slowly drop it into a round bottom flask with a constant pressure funnel. After the dropwise addition, spin dry with a rotary evaporator, and then place it in a vacuum drying oven at 70°C for 24 hours to obtain N,N-dimethyl Aminomethylferrocene and trinitrotoluene eutectic powder, its structural characterization data are as follows:
[0033] FR-IR (KBr, cm -1 ): 3676, 3195, 1148, 1541, 1471, 815; 1 H NMR (400MHz, DMSO) δ: 9.03(s, 2H), 4.23(t, 9H), 3.82(s, 2H), 3.52(s, 3H), 2.56(s, 6H); 13 C NMR (600 MHz, DMSO) δ: 161.29, 142.35, 125.66, 124.67, 75.31, 71.44...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com