Method for determining chloride ion content in polyhalite
A technology for chloride ion content and determination method, which is applied in the field of photometric determination of chloride ion content in polyhalite, can solve problems such as large analysis errors and expensive ion chromatography, and achieve high precision, stable results, and wide applicability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
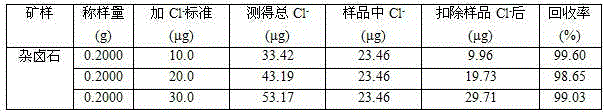
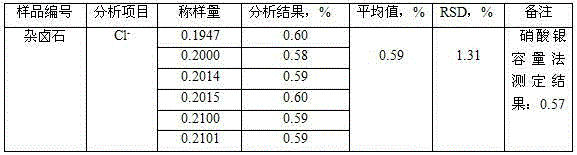
[0018] Embodiment 1, the determination method of chloride ion content in a kind of polyhalite: dissolve polyhalite sample with acid, in acid medium, chloride ion reacts with mercury thiocyanate, thiocyanate ion is replaced, and add The iron III ion in the iron salt of the solution forms a red complex, and the absorbance of the complex in the test solution is measured at 460nm of the spectrophotometer, thereby calculating the content of chloride ion in the polyhalite.
Embodiment 2
[0019] Embodiment 2, a kind of assay method of chloride ion content in polyhalite, its concrete steps are as follows:
[0020] (1) Weigh 0.2g polyhalite sample, accurate to 0.0001g, put it in a 250mL beaker, add 5mL of nitric acid solution, heat and boil to dissolve, and keep for 3min; the nitric acid solution is AR grade, and the nitric acid solution is in a volume ratio of 1: 1 of concentrated nitric acid mixed with water;
[0021] (2) Remove it after cooling, transfer it into a 250mL volumetric flask, release it with water to the mark, and shake well;
[0022] (3) Dispense 5mL of the test solution into a 10mL colorimetric tube with a stopper, add a small amount of water, add 2mL of ferric ammonium sulfate solution, and mix well; add 1mL of mercury thiocyanate-ethanol solution, mix well; leave it at room temperature for 10min; The mass concentration of the described ferric ammonium sulfate solution is 60g / L; the mass concentration of the described mercury thiocyanate-ethano...
Embodiment 3
[0025] Embodiment 3, a kind of assay method of chloride ion content in polyhalite, its concrete steps are as follows:
[0026] (1) Weigh 0.5g of polyhalite sample, accurate to 0.0001g, put it in a 250mL beaker, add 20mL of nitric acid solution, heat and boil to dissolve, and keep for 3-5min; the nitric acid solution is AR grade, and the nitric acid solution is divided by volume. 1:4 concentrated nitric acid mixed with water;
[0027] (2) Remove it after cooling, transfer it into a 250mL volumetric flask, release it with water to the mark, and shake well;
[0028] (3) Dispense 10 mL of the test solution into a 50 mL colorimetric tube with a stopper, add a small amount of water, add 2 mL of ferric ammonium sulfate solution, and mix well; add 1 mL of mercury thiocyanate-ethanol solution, and mix well; leave at room temperature for 30 minutes; The mass concentration of the described ferric ammonium sulfate solution is 60g / L; the mass concentration of the described mercury thiocya...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com