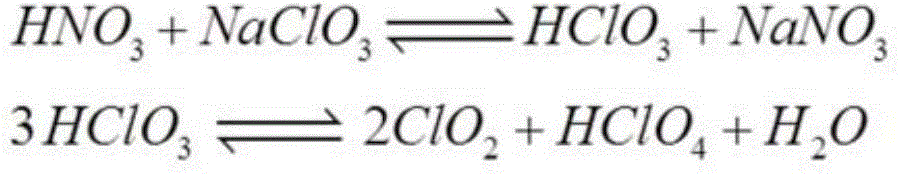Method of production of graphite oxide and uses thereof
一种石墨、用途的技术
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: Synthesis of graphite oxide from sulfate-intercalated graphite flakes by the method of the present invention
[0106] A mixture of 2.0 g of sulfate intercalated sheets (ASBURY 1721: 300 μm) and 30 ml of nitric acid (Merck 100% fuming nitric acid) was cooled to 0° C. in a 100 ml glass reactor. A purge flow of 250 liters / hour of air was initiated through the reactor and maintained throughout the reaction. Acid vapor is visible at room temperature, but is no longer visible at 0°C.
[0107] Then 17.0 g of sodium chlorate (oxidizing agent) was added in 1 g portions over 2 hours while stirring. Green vapour appeared after adding a few grams, indicating ClO 2 The presence. will contain the formed ClO 2 The gaseous effluent of C is continuously neutralized by blowing into sodium bisulfite solution (about 40%), and then the gaseous chlorine produced by this neutralization step is neutralized by sodium hydroxide before being released to the atmosphere.
[0108] Th...
Embodiment 2
[0111] Example 2: Synthesis of graphite oxide by standard Brodie method
[0112] In order to compare the graphite oxide obtained by the method of the present invention and the known methods of preparing graphite oxide, the standard Brodie method was applied to graphite powder as follows: 2.0 g (0.16 moles) of graphite powder (Timcal SFG6) and 17.0 g (0.16 moles) of chlorine The sodium mixture was cooled to -20°C in a 50ml Schlenk tube. A 15 ml volume (2.78 moles) of nitric acid (Merck 100% fuming nitric acid) was added slowly over 2 hours. At the beginning, only acid vapour is visible, after a few milliliters the vapour turns green, indicating ClO 2 The presence. The reaction was then allowed to reach room temperature over 12 hours. The color of the mixture turned turquoise during the previous 12 hours. The mixture was warmed to 60°C over 1 hour 30 minutes and held for 30 minutes. During heating, ClO 2 The release is especially severe (b.p.ClO 2 11°C). The reaction was...
Embodiment 3
[0115] Example 3: Synthesis of graphite oxide by standard Staudenmaier method (comparative example: non-inventive)
[0116] The Staudenmaier method as described in Staudenmaier, 1898 (see above) was applied to graphite powder (Timcal SFG6). The resulting graphite oxide was of poor quality, with a very low degree of oxidation (C / O ratio of 9.8-12.8). These results indicate that the staudenmaier method is not suitable for the large-scale production of graphite oxide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of oxidation | aaaaa | aaaaa |
| degree of oxidation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com