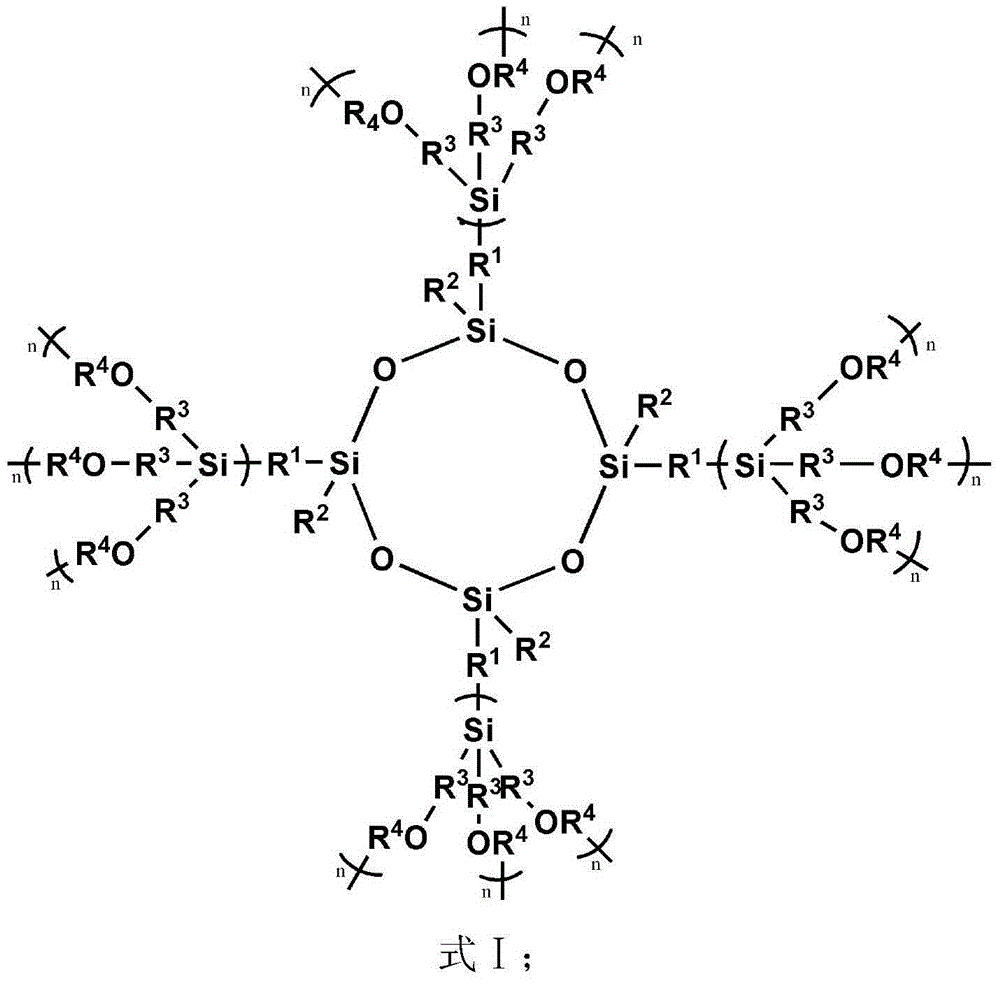
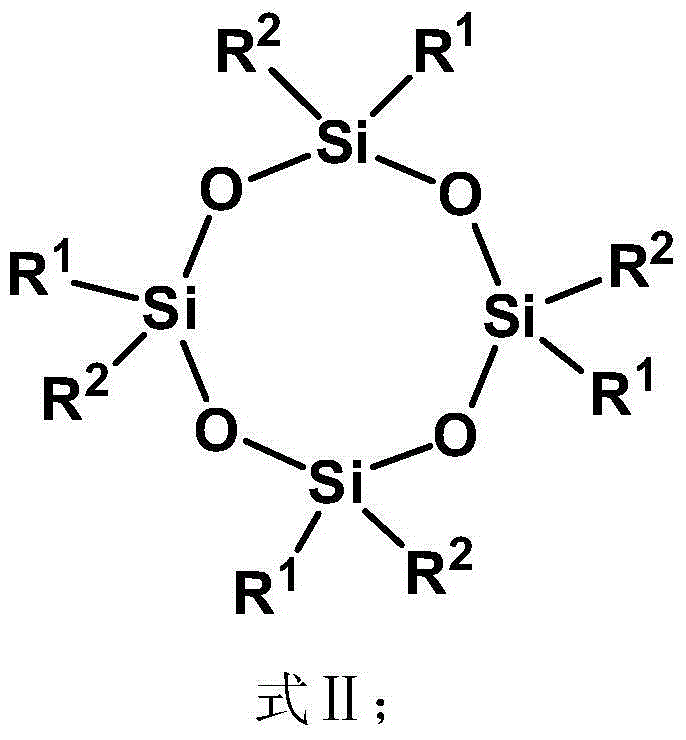
Spherical dendritic organic silicon macromolecule, preparation method thereof and application
A dendritic and silicone technology, applied in the direction of coating, etc., can solve the problem of low collision probability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] (1) 5.14g tetravinyl cyclotetrasiloxane (D 0 ) (Tetramethyltetravinylcyclotetrasiloxane, R 1 is methyl, R 2 is vinyl), 7.09g methyldichlorosilane (A) and 0.1g Karstedt catalyst (C) were dissolved in 300mL tetrahydrofuran (B) and mixed uniformly, wherein the amount of Karstedt catalyst was passed through tetravinyl cyclotetrasiloxane The molar mass of vinyl is calculated, and the molar mass of platinum is 1×10 of vinyl -4 . Reacted for 24h under normal temperature conditions, by 1 H-NMR to monitor the reaction. Excess unreacted methyldichlorosilane and solvent were removed by rotary evaporator under reduced pressure. The catalyzer is settled by pentane, and finally the low-boiling substance is removed by vacuumizing to obtain 11.0 g of colorless oily liquid (D 1X ), productive rate 90%; The product D obtained 1X The edge of contains 8 active groups Cl, paving the way for the subsequent reaction;
[0081] (2) Add 11.0g of D 1X and 12.71g of tetramethylethylenedia...
Embodiment 2
[0084] (1) 5.14g tetravinyl cyclotetrasiloxane (D 0 ) (Tetramethyltetravinylcyclotetrasiloxane, R 1 is methyl, R 2 is vinyl), 8.07g trichlorosilane (A) and 0.1g Karstedt catalyst (C) are dissolved in 300mL tetrahydrofuran (B) and mixed uniformly, wherein the amount of Karstedt catalyst is determined by vinyl in tetravinylcyclotetrasiloxane The molar weight of platinum is calculated as the molar weight of the vinyl group is 1×10 -4 . Reacted for 24h under normal temperature conditions, by 1 H-NMR to monitor the reaction. Excess unreacted trichlorosilane and solvent were removed by rotary evaporator under reduced pressure. The catalyzer is settled by pentane, and finally the low-boiling material is removed by vacuumizing to obtain 11.9 g of colorless oily liquid (D 1X ), productive rate 90%, the product D that obtains 1X The edge contains 12 active groups Cl, paving the way for the subsequent reaction;
[0085] (2) Add 11.9g of D 1X and 20.61g of tetramethylethylenediam...
Embodiment 3
[0088] (1) 5.14g tetravinyl cyclotetrasiloxane (D 0 ) (Tetramethyltetravinylcyclotetrasiloxane, R 1 is methyl, R 2 is vinyl), 7.09g methyldichlorosilane (A) and 0.1g Karstedt catalyst (C) were dissolved in 300mL tetrahydrofuran (B) and mixed uniformly, wherein the amount of Karstedt catalyst was passed through tetravinyl cyclotetrasiloxane The molar mass of vinyl is calculated, and the molar mass of platinum is 1×10 of vinyl -3 . Reaction 72h under normal temperature condition, by 1 H-NMR to monitor the reaction. Excess unreacted methyldichlorosilane and solvent were removed by rotary evaporator under reduced pressure. The catalyzer is settled by pentane, and finally the low-boiling substance is removed by vacuumizing to obtain 11.0 g of colorless oily liquid (D 1X ), productive rate 90%; The product D obtained 1X The edge contains 8 active groups Cl, paving the way for the subsequent reaction;
[0089] (2) Add 11.0g of D 1X and 12.71g of tetramethylethylenediamine (T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com