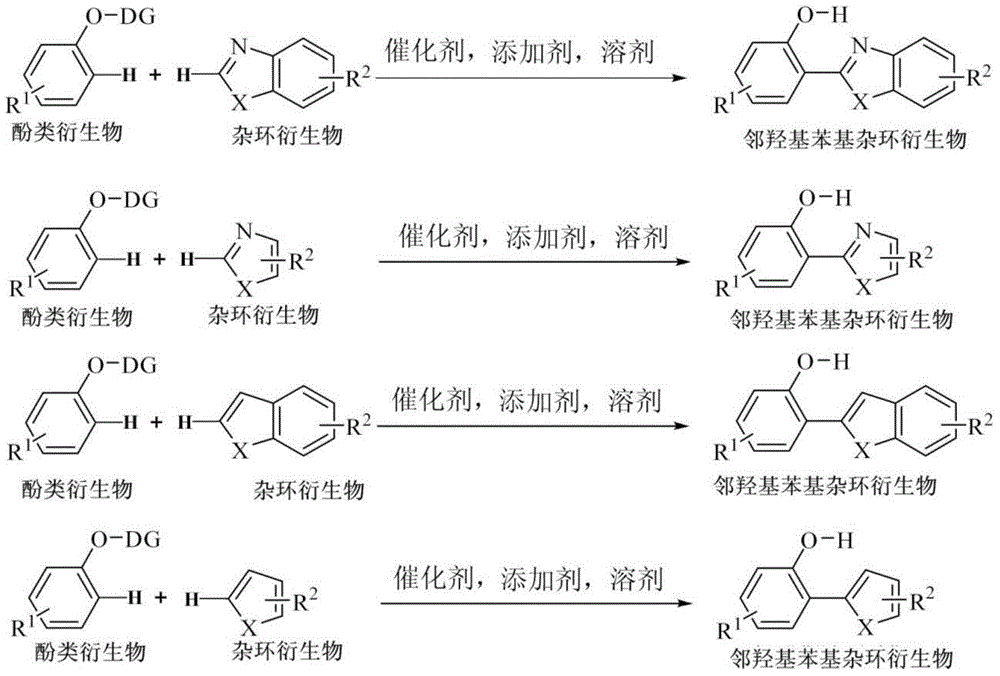
Method for high-efficiency preparation of an o-hydroxyphenyl heterocyclic derivative through C-H/C-H oxidative coupling reaction based on transition metal catalysis
A technology of oxidative coupling reaction and transition metal catalysis, applied in the direction of steroids, organic chemistry, etc., can solve such problems that cannot be completed, and achieve the reduction of total cost, increase of total yield, improvement of atom economy and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
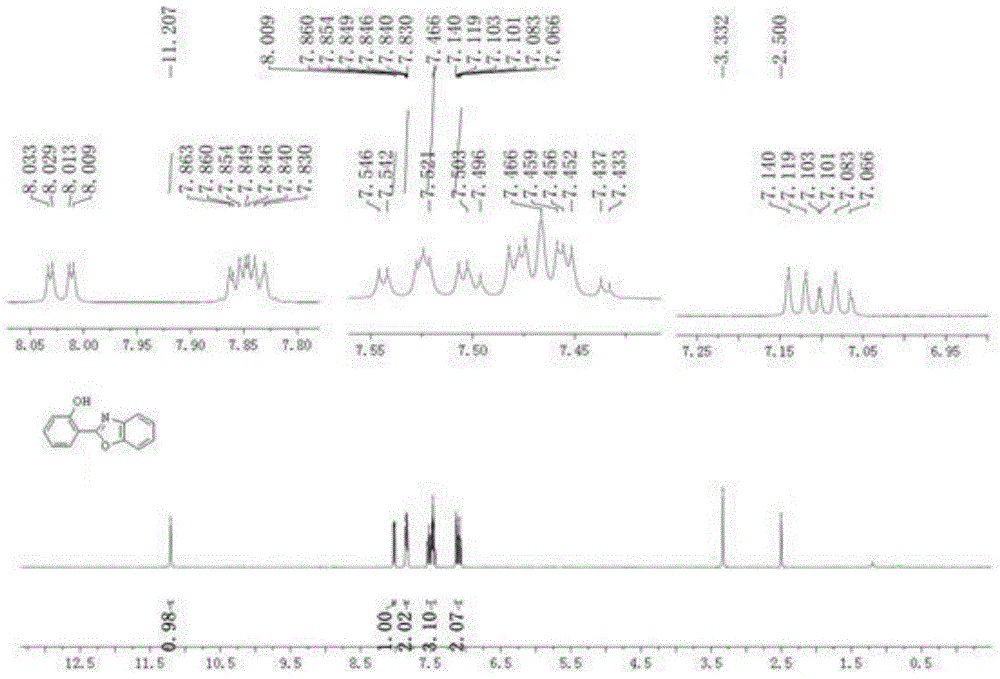
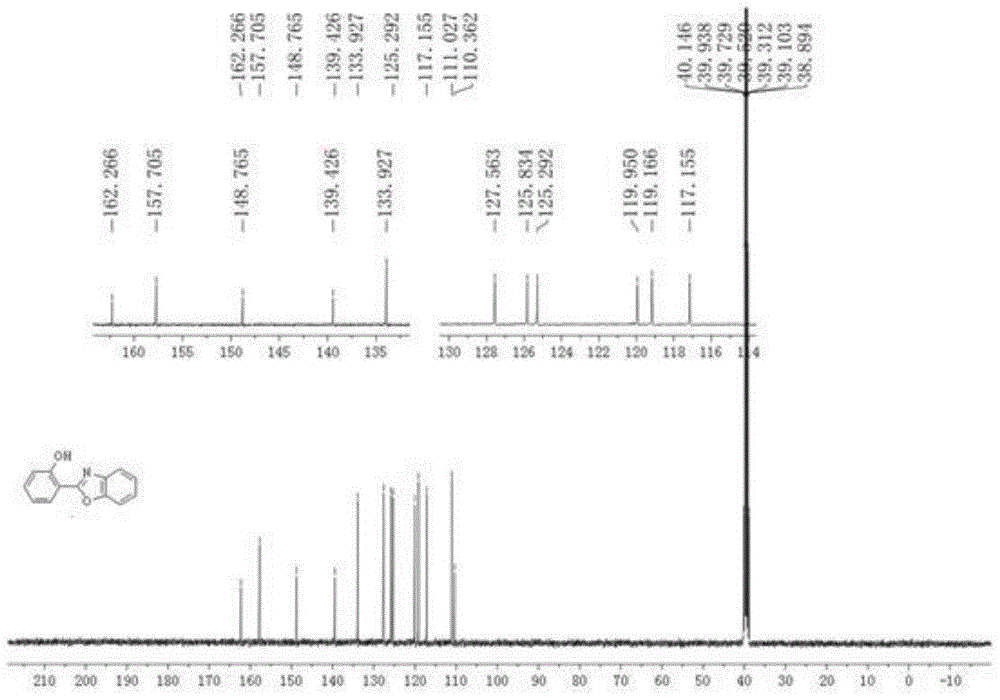
[0028] Embodiment 1: the synthesis of o-hydroxyphenylbenzoxazole
[0029] (1) Phenoxyacetimide (30.2 mg, 0.2 mmol), benzoxazole (36 mg, 0.3 mmol), dichloro(pentamethylcyclopentadienyl) rhodium(III) dimer ( 2.5 mol%, 3.2 mg, 5 μmol), silver hexafluoroantimonate (10 mol%, 6.8 mg, 20 μmol), silver carbonate (0.2 equiv, 11 mg, 40 μmol), pivalic acid (2.0 equiv, 41 mg , 0.4 mmol), cesium pivalate (80 mol%, 38 mg, 0.16 mmol), N,N -Dimethylformamide (1 mL), stirred evenly under anhydrous and oxygen-free conditions, heated to 140 ° C, and reacted for 24 hours;
[0030] (2) After the reaction is completed, cool the reaction tube to room temperature, add ethyl acetate to dilute the reaction system, then filter through diatomaceous earth, and wash with ethyl acetate, combine the filtrates, wash with saturated saline, separate liquid, anhydrous sodium sulfate After drying, the solvent was removed under reduced pressure, and the residue was separated and purified by silica gel column ...
Embodiment 2
[0031] Embodiment 2: the synthesis of 2-(5-chlorobenzoxazolyl-2-)phenol
[0032] (1) Phenoxyacetimide (30.2 mg, 0.2 mmol), 5-chlorobenzoxazole (46 mg, 0.3 mmol), dichloro(pentamethylcyclopentadienyl)rhodium(III) di polymer (2.5 mol%, 3.2 mg, 5 μmol), silver hexafluoroantimonate (10 mol%, 6.8 mg, 20 μmol), silver carbonate (0.2 equiv, 11 mg, 40 μmol), pivalic acid (2.0 equiv , 41 mg, 0.4 mmol), cesium pivalate (80 mol%, 38 mg, 0.16 mmol), N,N -Dimethylformamide (1 mL), stirred evenly under anhydrous and oxygen-free conditions, heated to 140 ° C, and reacted for 24 hours;
[0033] (2) After the reaction is completed, cool the reaction tube to room temperature, add ethyl acetate to dilute the reaction system, then filter through diatomaceous earth, and wash with ethyl acetate, combine the filtrates, wash with saturated saline, separate liquid, anhydrous sodium sulfate After drying, the solvent was removed under reduced pressure, and the residue was separated and purified by ...
Embodiment 3
[0034] Embodiment 3: the synthesis of compound benzoxazole substituted estrone
[0035] (1) Estrone acetimide (65 mg, 0.2 mmol), benzoxazole (36 mg, 0.3 mmol), dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (2.5 mol%, 3.2 mg, 5 μmol), silver hexafluoroantimonate (10 mol%, 6.8 mg, 20 μmol), silver carbonate (0.2 equiv, 11 mg, 40 μmol), pivalic acid (2.0 equiv, 41 mg, 0.4 mmol), cesium pivalate (80 mol%, 38 mg, 0.16 mmol), N,N -Dimethylformamide (1 mL), stirred evenly under anhydrous and oxygen-free conditions, heated to 140 ° C, and reacted for 24 hours;
[0036](2) After the reaction is completed, cool the reaction tube to room temperature, add ethyl acetate to dilute the reaction system, then filter through diatomaceous earth, and wash with ethyl acetate, combine the filtrates, wash with saturated saline, separate liquid, anhydrous sodium sulfate After drying, the solvent was removed under reduced pressure, and the residue was separated and purified by silica ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com