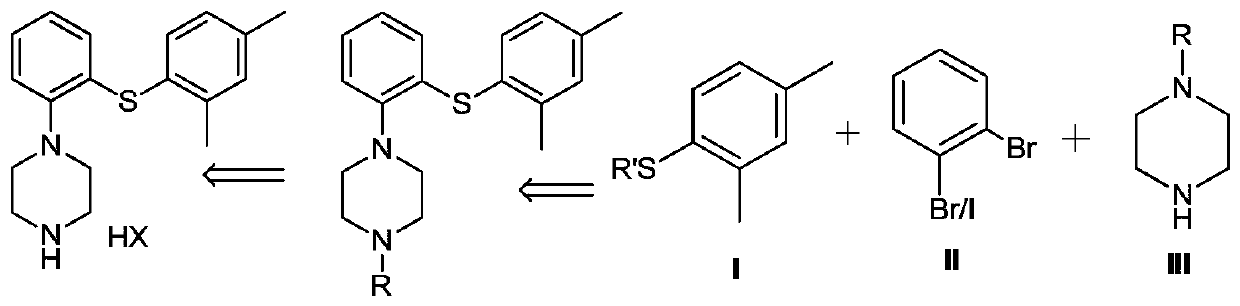
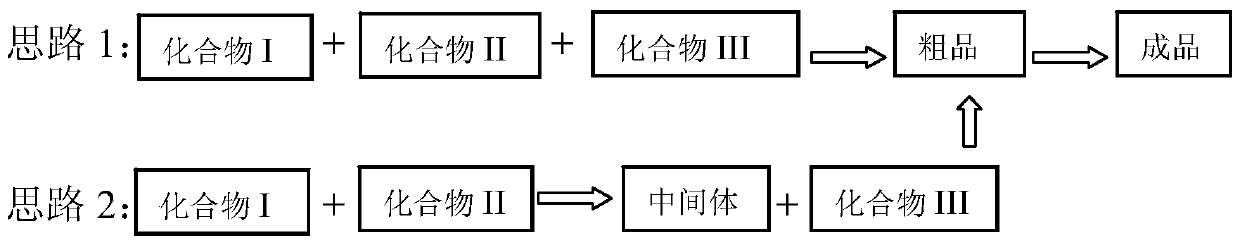
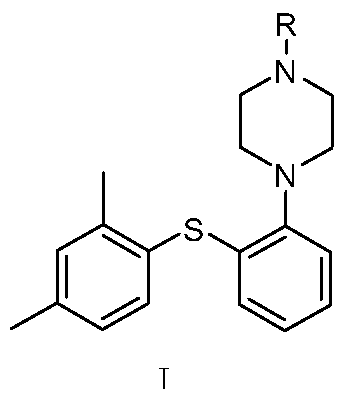
Preparation method of diaryl sulfide amine compounds
A technology of dimethylphenylsulfanyl and phenyl, which is applied in the field of preparation of 1-[2-phenyl]piperazine derivatives, can solve problems such as difficult purification and coupling impurities, and achieve high sample purity , low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]Example 1: Synthesis of 2-{4-[2-(2,4-dimethylphenylsulfanyl) phenyl] piperazin-1-yl} ethyl acetate
[0061]
[0062] Add 28.5g (0.16mol) of bis(2-chloroethyl)amine hydrochloride and 200ml of ethanol to a 500ml three-necked flask, heat up to 80°C, stir for 1 hour, and add 21.7g (0.17mol) of 2-chloroaniline , anhydrous sodium carbonate 7.4g (0.07mol), continue to heat and stir the reaction for 25h, cool to room temperature, filter with suction, the filtrate is concentrated to dryness, add acetonitrile 100ml for crystallization, and obtain 35.8g of white crystals, the yield is 96%.
[0063] Dissolve 14.0g (0.06mol) of 1-(2-chlorophenyl)piperazine hydrochloride in 100ml of anhydrous methanol, add dropwise saturated sodium bicarbonate solution to adjust the pH to 8-9 while stirring, cool, and filter with suction. The filtrate was concentrated to dryness to obtain a white solid, namely 1-(2-chlorophenyl)piperazine.
[0064] Dissolve the above solid in 100ml N,N-dimethylfor...
Embodiment 2
[0066] Embodiment 2: the synthesis of 1-[2-(2,4-dimethylphenylsulfanyl) phenyl] piperazine hydrobromide
[0067]
[0068] Add 28.5g (0.16mol) of bis(2-chloroethyl)amine hydrochloride and 200mL of butanol into a 500ml three-necked flask, heat up to 80°C, stir for 1h, add 29.2g (0.17mol) of 2-bromoaniline ) and anhydrous potassium carbonate 9.7g (0.07mol), continue to insulate and stir the reaction for 25h, cool to room temperature, filter with suction, concentrate the filtrate to dryness, add 100ml of acetonitrile for crystallization, and obtain 42.2g of white crystals with a yield of 95%.
[0069] Dissolve 16.6g (0.06mol) of 1-(2-bromophenyl)piperazine hydrochloride in 100ml of absolute ethanol, add a saturated sodium bicarbonate solution dropwise under stirring to adjust the pH to 8-9, cool, and filter with suction. The filtrate was concentrated to dryness to obtain a solid which was 1-(2-bromophenyl)piperazine.
[0070] Dissolve the above solid in 100ml of absolute ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com