DC cell based on HBV-HCV antigen, targeting immune cell population, preparation method and applications thereof
A cell and antigen technology, applied to other methods of inserting foreign genetic materials, cells modified by introducing foreign genetic materials, animal cells, etc., can solve the problems of immune cell preparation methods that need to be improved, to solve the problem of cytotoxicity and efficiency High, high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1. Materials and equipment
[0066] 1. Laboratory level: GLP
[0067] 2. Instrument and equipment:
[0068] Minus 80 degrees ultra-low temperature refrigerator (Zhongke Meiling, DW-HL388); ordinary refrigerator (Haier, double door, -20℃ and 4℃); mold incubator (Shanghai Boxun, MJX-160B-Z); constant temperature water bath ( SSW-420-2S); CO 2 Incubator (HF240); low-speed centrifuge (Shanghai Anting, TDL-40C); inverted biological microscope (Kexin, model: BLD-1); biological safety cabinet (Suzhou purification, double); electric pipette (German general RAND) 1-10ml; Micro pipette (Dalong) 1 set, 4 pieces; Program cooling box (Thermo); Liquid nitrogen tank (Jinfeng, YDS-120-216); PCR machine; Gel electrophoresis imaging system ; Electrophoresis instrument; Thermostat;
[0069] 3. Cell culture consumables:
[0070] Cell culture bag (Takara company); 75cm 2 Culture flask, 175cm 2 Culture flask, 1.8ml cryopreservation tube, 1.5ml EP tube, 50ml centrifuge tube, 15ml centrifuge tube, al...
Embodiment 2
[0132] Example 2: Clinical treatment
[0133] The CTL cells prepared in Example 1 were returned to the patient, and the effect of the infusion was observed, as follows:
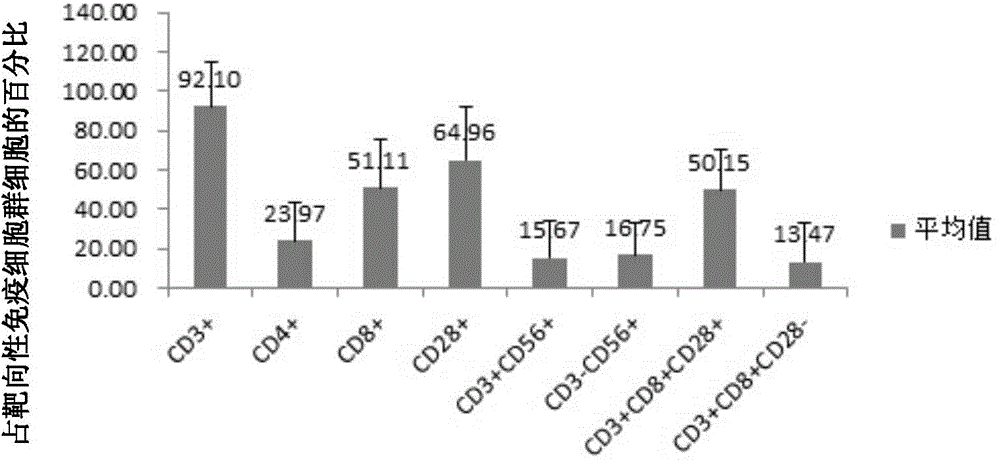
[0134] Twenty HBV-HCV-positive liver cancer patients were divided into 2 groups (10 in group A and 10 in group B). For group A, sufficient CTLs were obtained on the 14th and 15th day according to the method in Example 1. After the cells are transfused back to the patient, the amount of cells transfused each time is 1~2*10 9 ; Group B does not return cells (as a control). Blood is collected once and infused twice continuously as a course of treatment. After 3 courses of treatment, observe.
[0135] The results showed that there was a clear difference between group A and group B. All patients in group A felt less physical exhaustion, increased appetite, reduced pain, and weight gain after reinfusion. Among them, 6 patients had tumor indicators decreased; and The symptoms of group B did not improve. It can be clear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com