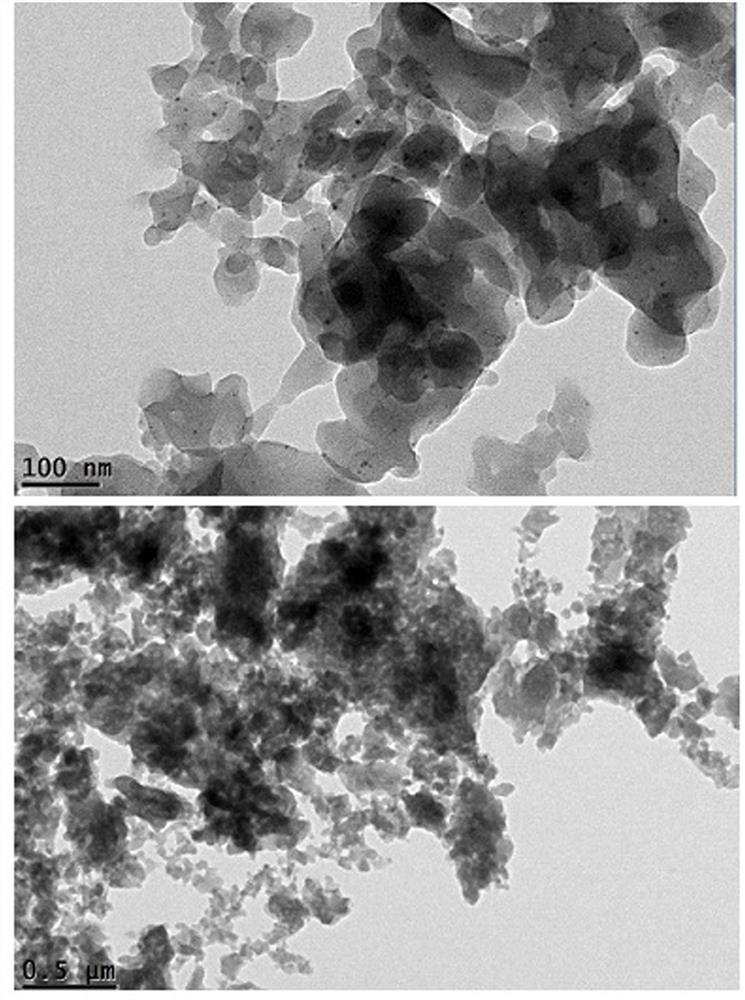
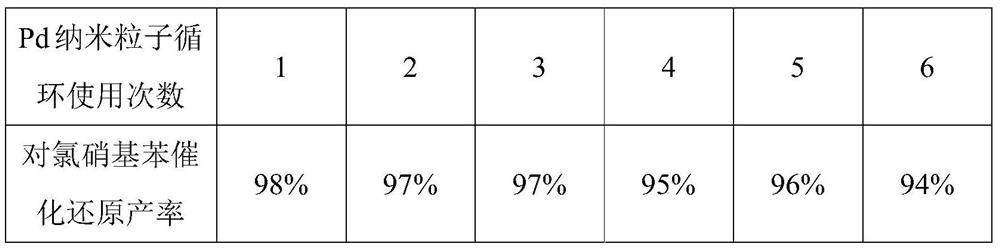
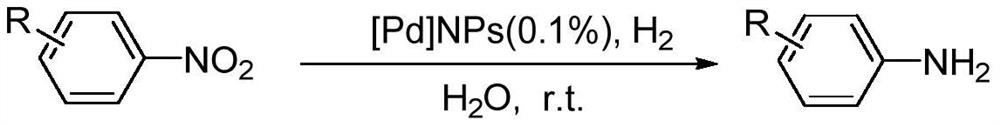A method for preparing PD nanoparticles
A nanoparticle and aniline technology, applied in the field of preparing Pd nanoparticles, can solve the problems of unfavorable recovery and reuse, difference in substrate adaptability, easy pollution of the environment, etc., and achieve the effect of low catalyst consumption, simple operation and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation method of Pd nanoparticles
[0017] (1) First add 2ml of ethanol solvent to the 10ml reaction bottle, then add 5mmol of aniline, 12.5mmol of tetrafluoroboric acid and mix evenly, add 12.5mmol of tert-butyl nitrite dropwise to the mixed solution under ice bath conditions, return to room temperature, and react After 0.5h, a small amount of diethyl ether was added to the reaction solution, and a white precipitate was formed, filtered and dried to obtain 0.92g (4.8mmol) of phenyldiazonium tetrafluoroborate, with a yield of 96%;
[0018] (2) Add 4mmol phenyldiazonium tetrafluoroborate (dissolved in 50ml tetrahydrofuran) into a 250ml reaction flask, then add 1mmol acetic acid Pd (dissolved in 50ml methanol), stir at room temperature for 10min~15min, the reaction solution turns orange , add dropwise 5mmol sodium borohydride (dissolved in 50ml methanol) solution under ice-bath conditions, and react at room temperature for 1h until the reaction is complete; the react...
Embodiment 2
[0020] Catalytic hydrogenation of p-nitrobenzene with Pd nanoparticles
[0021] In the 25ml reaction bottle, add 2mmol nitrobenzene, 10ml water is made solvent, 2.4mg Pd nanoparticles prepared by the present invention, feed H under normal temperature and pressure. 2 , after reacting for 12h, TLC detects that the reaction is complete, the reaction solution is extracted with dichloromethane, and the organic phase is extracted with anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and purified by column chromatography to obtain 0.18 g (1.94 mmol) of the corresponding target compound aniline, with a yield of 97%. analyze data: 1 H NMR (400MHz, CDCl 3 )δ=7.11(t, J=8.0Hz, 2H), 6.72(t, J=8.0Hz, 21H), 6.59(d, J=8.0Hz, 2H), 3.53(s, br, 2H); GC- MS,m / z,93[M + ].
Embodiment 3
[0023] Catalytic hydrogenation of p-chloronitrobenzene with Pd nanoparticles
[0024] In the 25ml reaction flask, add 2mmol p-chloronitrobenzene, 10ml water as solvent, 3mg of Pd nanoparticles prepared by the present invention, feed H under normal temperature and pressure. 2 , after reacting for 12h, TLC detects that the reaction is complete, the reaction solution is extracted with dichloromethane, and the organic phase is extracted with anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and purified by column chromatography to obtain 0.25 g (1.96 mmol) of the corresponding target compound p-chloroaniline, with a yield of 98%. The analysis data is: 1 H NMR (400MHz, CDCl 3 )δ=7.07(d, J=8.0Hz, 2H), 6.54(d, J=8.0Hz, 2H), 3.62(s, 2H); GC-MS, m / z, 127[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com