Flavour additives
A technology of amino acid and furanone, applied in the field of flavor additives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
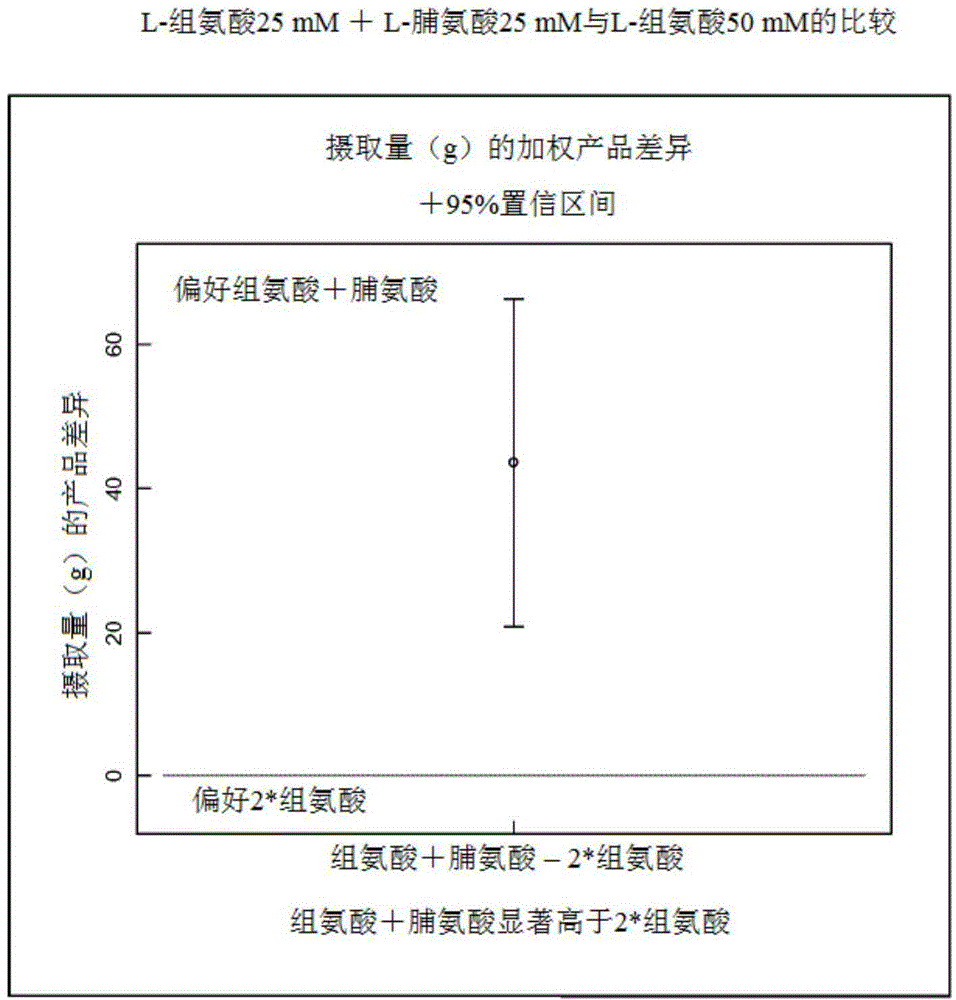
[0071] Cats were exposed to water containing 25 mM proline + 25 mM histidine with a composition containing 50 mM histidine. The method uses a two-bottle selection test with 24 cats (final number of cats may vary per test due to discarded data due to spillage, etc.). The cats were each in a separate house during the test periods and had free access to available water between test periods. The test consisted of a choice test between a tastant / mixture dissolved in deionized water at a given concentration and deionized water alone or another tastant / mixture. Position bias (eg A / B Exposure 1 and B / A Exposure 2) and evaporation losses are controlled. The testing time was 36 hours (ie, 18 hours per day, alternated every two days). After each test on two consecutive days, the cats were allowed to rest for two consecutive days. Cats were offered a dry diet as a single meal 1 hour after the start of the test period, calculated to meet the individual needs of each cat.
[0072] The r...
example 2
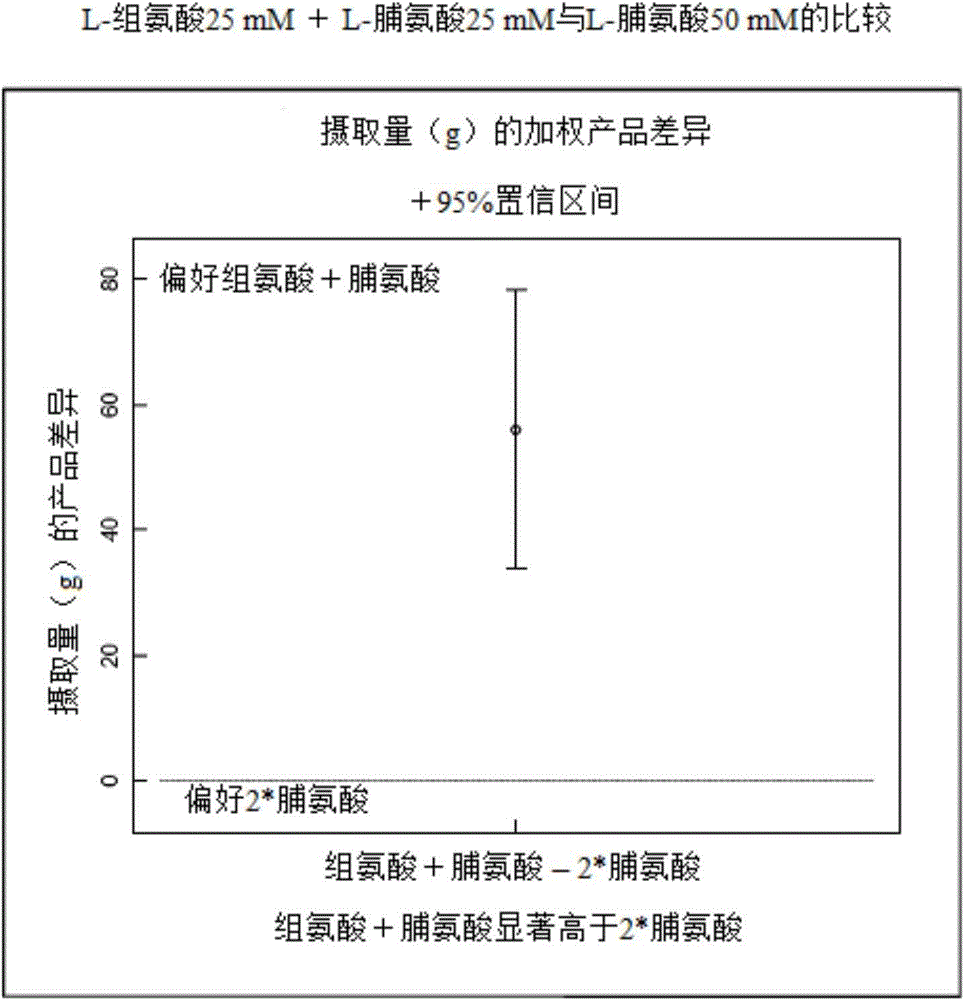
[0080] The difference test was performed as in example 1, however, the composition comprising 25 mM proline + 25 mM histidine was compared to the composition comprising 50 mM proline only.
[0081] The result is shown below and figure 2 middle.
[0082] Intake (g) analysis
[0083] ANOVA table for fixed effects
[0084]
[0085]
[0086] *0.0000 means a number less than 0.0001
[0087] Table of Mean Product Differences, Standard Errors and 95% Confidence Intervals
[0088]
[0089] It can be seen that the intake of proline + histidine was on average 56.07 g higher than the intake of proline alone, ie animals clearly preferred the combination of proline and histidine over proline alone.
example 3
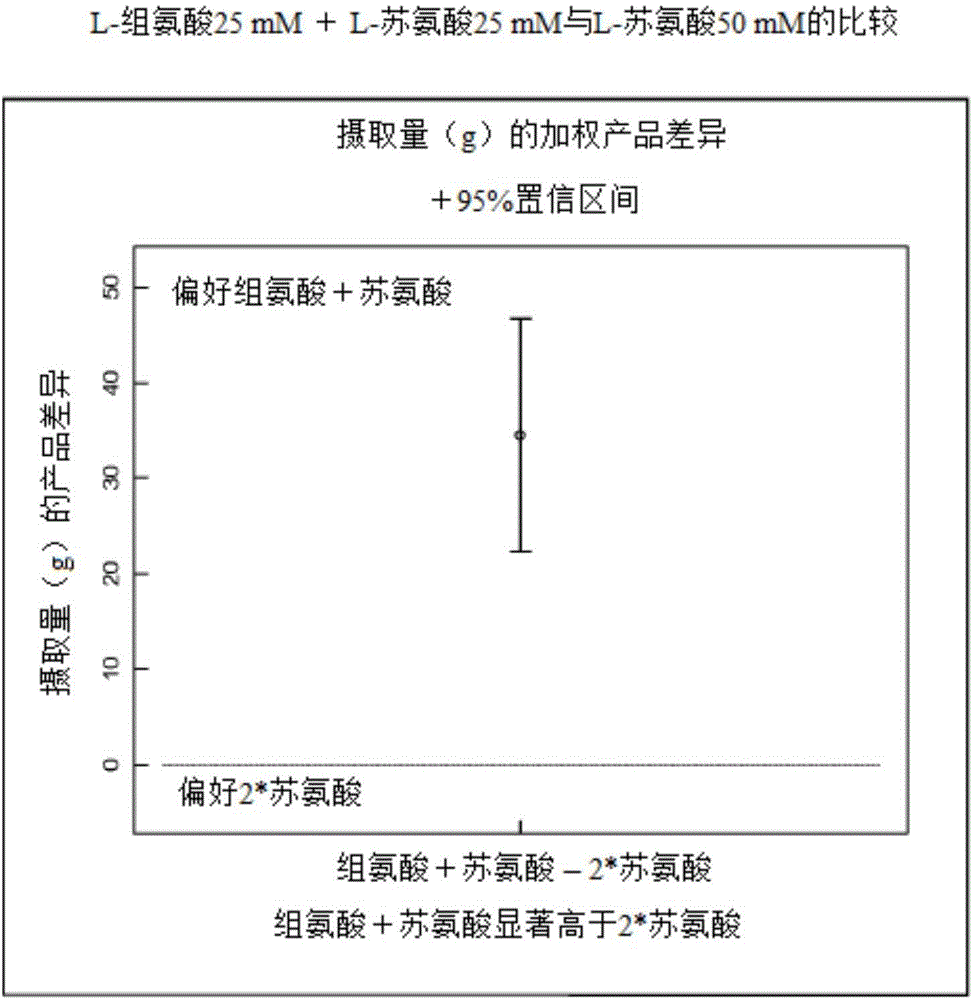
[0091] The difference test was performed as in Example 1, however, a composition comprising 25 mM threonine + 25 mM histidine was compared to a composition comprising 50 mM threonine only.
[0092] The result is shown below and image 3 middle.
[0093] Intake (g) analysis
[0094] ANOVA table for fixed effects
[0095]
[0096] Table of Mean Product Differences, Standard Errors and 95% Confidence Intervals
[0097]
[0098] It can be seen that the intake of threonine+histidine is 34.48g higher on average than the intake of threonine alone, that is, animals clearly prefer the combination of threonine and histidine compared to threonine alone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com