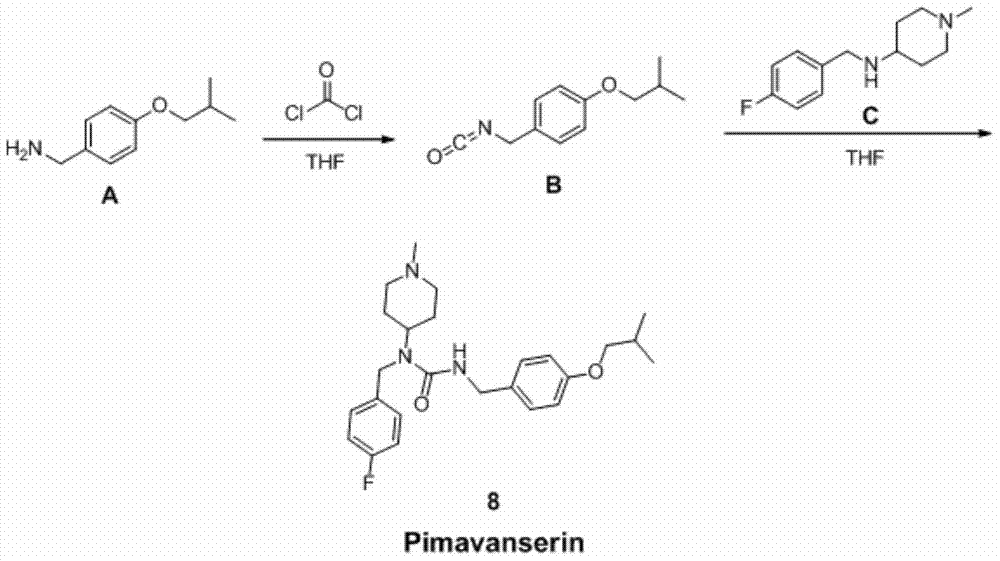
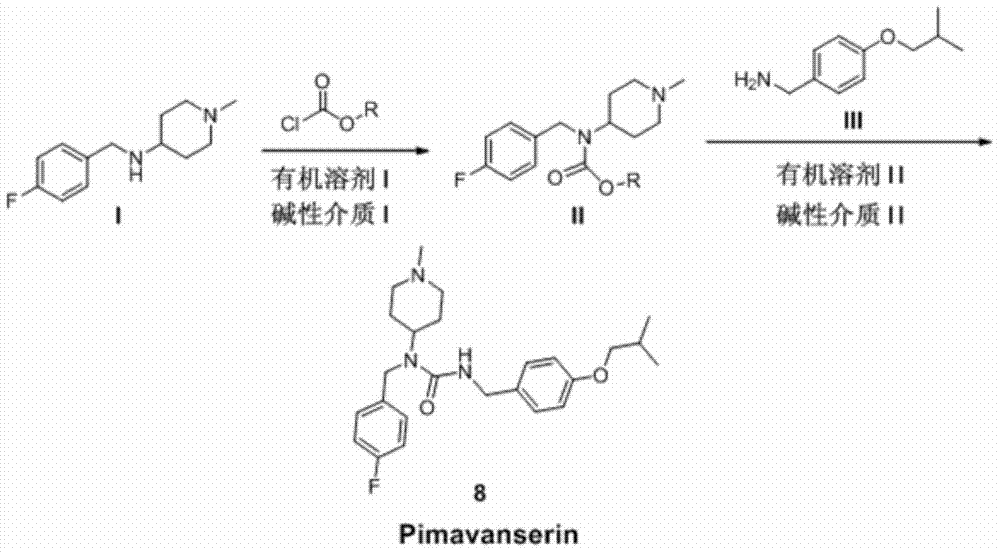
Preparation method of Pimavanserin
A technology of pimavanserin and compounds, which is applied in the field of preparation of Parkinson's disease drug pimavanserin, can solve the problem of difficult mass production control, inability to control the quality of raw materials and intermediates, and unsuitability for industrial production and other problems, to achieve the effect of mild reaction conditions, easy quality control and impurity analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
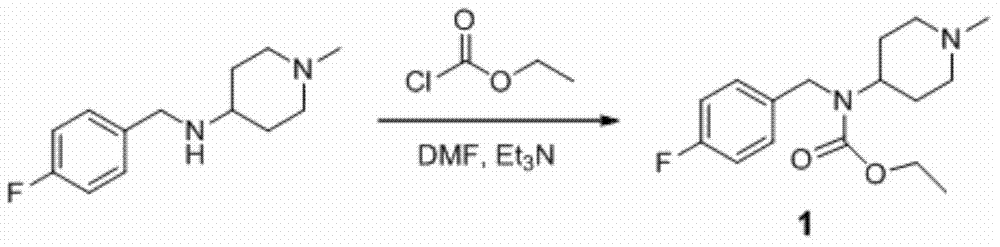
[0035] Preparation of 4-fluorobenzyl(1-methylpiperidin-4-yl) ethyl carbonate (1)
[0036]
[0037] 4-(4-fluorobenzylamino)-1-methylpiperidine (22.2g, 0.1mol) was dissolved in N,N-dimethylformamide (30mL), triethylamine (21mL, 0.15mol, density 0.726), stirred, cooled to -10°C, added dropwise ethyl chloroformate (11.47mL, 0.12mol, density 1.14), the addition was completed in 1 hour, the reaction mixture was stirred at 25°C for 12 hours, and the reaction was monitored by HPLC until the raw material was less than 0.1% , cooled to 0°C, slowly added 300mL of water, stirred at 25°C for 12 hours, filtered with suction, and washed the solid with water (300mL×3) to obtain an off-white solid. The resulting solid was recrystallized with 95% ethanol (200 mL), suction filtered, and the solid was washed with 50% ethanol (50 mL×2) to obtain purified compound 1, a white solid, 20.00 g, purity 99.56%, yield: 68%; Product characterization: MS (m / z): 295.2 [MH + ].
Embodiment 2
[0039] Preparation of 4-fluorobenzyl(1-methylpiperidin-4-yl)isopropyl carbonate (2)
[0040]
[0041] 4-(4-Fluorobenzylamino)-1-methylpiperidine (22.2g, 0.1mol) was dissolved in acetonitrile (150mL), anhydrous sodium carbonate (15.9g, 0.15mol) was added, stirred, and cooled to 0°C , dropwise added isopropyl chloroformate (16.55mL, 0.12mol, density 0.892), the dropwise addition was completed in 1 hour, the reaction mixture was stirred at 25°C for 12 hours, the reaction was monitored by HPLC until the raw material was less than 0.1%, the temperature was lowered to 0°C, and 750mL of water, stirred at 60°C for 1 hour, filtered with suction, and washed the solid with water (300 mL×3) to obtain an off-white solid. The resulting solid was recrystallized with 95% ethanol (200 mL), filtered with suction, and the solid was washed with 50% ethanol (50 mL×2) to obtain purified compound 2, a white solid, 22.96 g, with a purity of 99.36%, and a yield of 74%; Product characterization: MS...
Embodiment 3
[0043] Preparation of 4-fluorobenzyl(1-methylpiperidin-4-yl)pentyl carbonate (3)
[0044]
[0045] 4-(4-fluorobenzylamino)-1-methylpiperidine (22.2g, 0.1mol) was dissolved in dichloromethane (600mL), and N,N-diisopropylethylamine (25mL, 0.15mol , density 0.782), stirred, cooled to 0°C, amyl chloroformate (17.42 mL, 0.12 mol, density 1.04) was added dropwise, and the addition was completed in 1 hour. The reaction mixture was stirred at 40°C for 3 hours, and the reaction was monitored by HPLC to less than 0.1% starting material. Cool down to 0°C, add 300mL of water, and stir at 25°C for 12 hours. The liquid was separated, and the aqueous phase was discarded. The dichloromethane phase was washed with a saturated aqueous sodium chloride solution (200 mL×3), dried over anhydrous magnesium sulfate, and concentrated to dryness to obtain a yellow solid. The solid was recrystallized with 95% ethanol (200mL), suction filtered, and the solid was washed with 50% ethanol (50mL×2) to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com