Sample method for synthesis of thianthrene based on sulfur powder
A technology of thianthrene and sulfur powder, which is applied in the field of environmentally friendly synthesis of thianthrene, achieving the effect of mild conditions and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
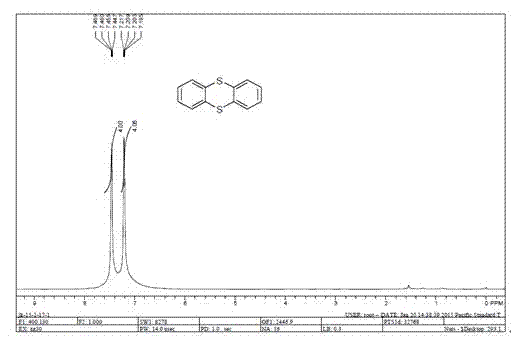
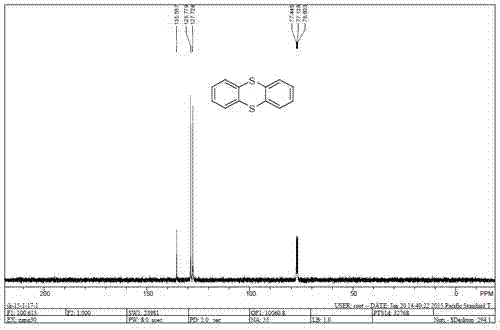
[0009] The present invention is achieved in that in a 25 mL round bottom flask, add o-diiodobenzene (0.5 mmol), sulfur powder (0.5 mmol), cuprous iodide (0.05 mmol), 1,10-phenanthroline (0.1 mmol), potassium carbonate (1.0 mmol) and dimethyl sulfoxide (2 mL). The resulting mixed system is then 90 o C was stirred for 12 hours. After cooling to room temperature, 10 mL of water was added to the system, then extracted with ethyl acetate (3×10 mL), and dried over anhydrous sodium sulfate. After distilling off the solvent, the obtained residue was subjected to silica gel column chromatography to obtain a pure product with a yield of 82%. The reaction formula and data are as follows figure 1 shown. The structure and purity of the obtained product were determined by proton nuclear magnetic resonance spectrum and carbon spectrum test and compared with literature data.
[0010] The proton nuclear magnetic resonance spectrum and carbon spectrum data of product thianthrene are:
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com