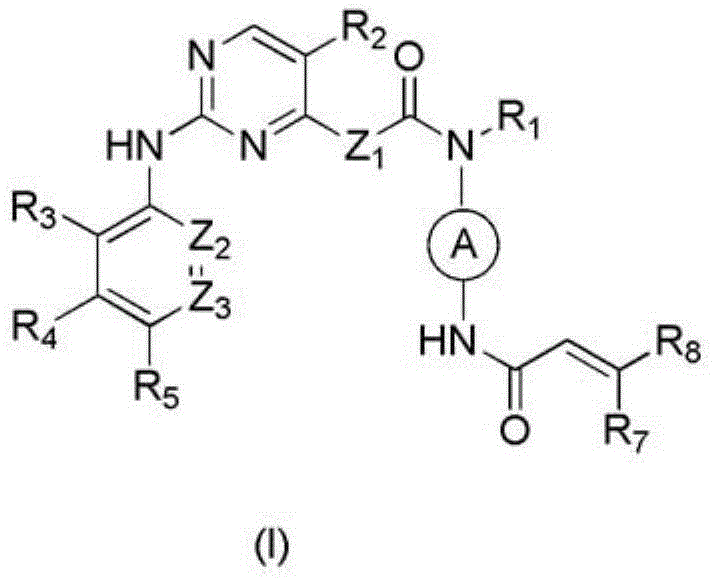
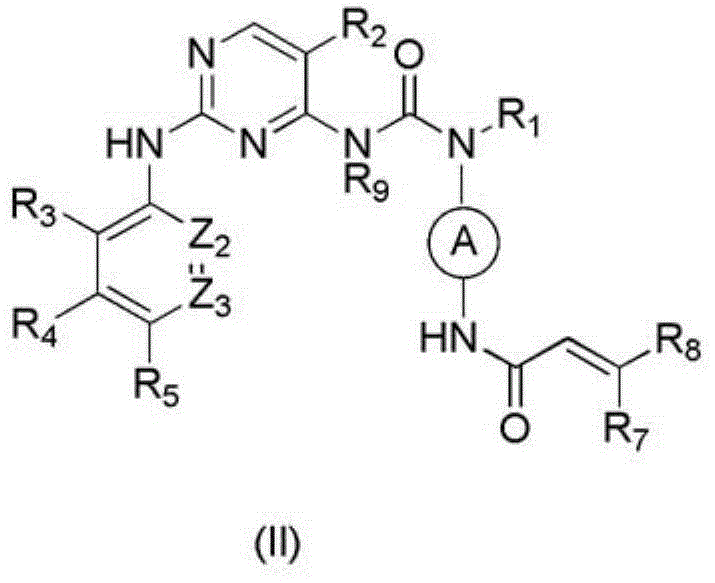
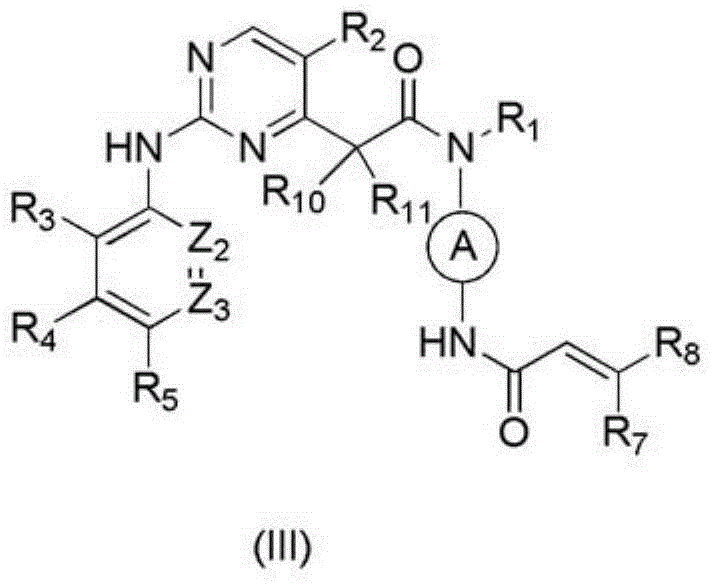
Arylaminopyrimidine compound and use thereof and pharmaceutical composition and medicinal composition prepared from arylaminopyrimidine compound
A technology for arylaminopyrimidines and compounds, which can be used in drug combinations, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as intolerable toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0173] Preparation of 3-amino-6-morpholinopyridine
[0174]
[0175] step 1:
[0176] At room temperature, 2-bromo-5-nitropyridine (2 g, 10 mmol) was added to 10 ml of morpholine solution, and vigorously stirred at room temperature for 4 hours. After the reaction, a yellow solid precipitated out. After filtration, the yellow solid was washed with 50 mL petroleum ether to give 4-(5-nitropyridin-2-yl)morpholine (1.9 g, 92%). Spectral data: MS m / z (ESI): 210.1 [M+H] +
[0177] Step 2:
[0178] At room temperature, palladium on carbon (100 mg, 10% wt) was added to 4-(5-nitropyridin-2-yl) morpholine (1 g, 9.1 mmol) in 60 ml of methanol solution, vigorously stirred at room temperature under a hydrogen atmosphere 20 hours. After the reaction, the palladium carbon was filtered off, and the filtrate was concentrated under reduced pressure to obtain the product 3-amino-6-morpholinylpyridine, which was directly used in the next reaction. Spectral data: MS m / z (ESI): 180.1 [M...
Embodiment 1
[0291] N-(3-(3-(2-((2-methoxy-4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin4-yl)ureido)phenyl)propene Preparation of amides (cpd-1)
[0292]
[0293] Step 1: Synthesis of 2-chloro-4-pyrimidine isocyanate
[0294] At 0 degrees Celsius, 4-amino-2-chloropyrimidine (4.8g, 37.0mmol) was added to a solution of triphosgene (5.5g, 18.5mmol) in 200ml of tetrahydrofuran (THF), and then N,N- - Diisopropylethylamine (DIEA) (6.23 g, 48.2 mmol). Stir vigorously at room temperature for 4 hours. The progress of the reaction was detected by TLC. After the substrate was completely reacted, the product 2-chloro-4-pyrimidine isocyanate was directly used in the next reaction.
[0295] Step 2: Synthesis of 1-(2-chloropyrimidin-4-yl)-3-(3-nitrophenyl)urea
[0296] At room temperature, triethylamine (TEA) (11 g, 110 mmol) and 3-nitroaniline (5.6 g, 40.3 mmol) were added to a solution of 2-chloro-4-pyrimidine isocyanate (5.7 g, 36.6 mmol) in 200 ml of THF , stirred vigorously at room temper...
Embodiment 2
[0303] N-(3-(3-(2-((4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)ureido)phenyl)acrylamide (cpd-2 ) preparation
[0304]
[0305] Step 1: Synthesis of 1-(2-((4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)-3-(3-nitrophenyl)urea
[0306] 4-(4-Methylpiperazin-1-yl)aniline (392 mg, 2.00 mmol) and trifluoroacetic acid (700 mg, 6.0 mmol) were added to 1-(2-chloropyrimidin-4-yl)-3-( 3-Nitrophenyl)urea (600mg, 2.00mmol) in 14ml of n-butanol was stirred vigorously at 130°C for 7 hours. After the reaction, the system was cooled to room temperature and filtered to obtain a brown solid. Wash the brown solid with 50 ml of ethanol to give compound 1-(2-((4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)-3-(3-nitro Phenyl)urea (500 mg), the product was used directly in the next step. Yield: 56%, purity: 72%, spectral data: MS m / z (ESI): 449.2 [M+H] +
[0307] Step 2: Synthesis of 1-(2-((4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)-3-(3-aminophenyl)urea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com