/-Borneol-alpha-D-glucopyranoside
A synthetic method and technology of borneoside, which is applied in the field of synthesis of borneoside, can solve the problems of no literature report, excess, heavy workload and other problems in the synthesis of borneoside
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
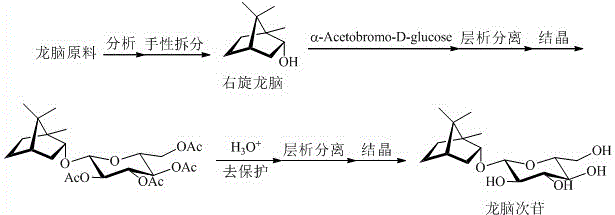
[0015] 1) Synthesis of compound III
[0016] Under the protection of argon, add 4.0 grams of (-)-Borneol, 2 grams of acetyl bromide-α-D-glucose, and an appropriate amount of anhydrous calcium chloride into dry dichloromethane, stir for 5 minutes, and then add 14 grams of silver carbonate , protected from light and stirred for 24 hours. The reaction solution was filtered, concentrated, and crudely separated by silica gel column chromatography to obtain the compound III The crude product, recrystallized with petroleum ether to obtain the compound of white needle crystal III , the yield is 24.0%.
[0017] 2) Synthesis of borneoside
[0018] Under the protection of argon, 3 g of compound III was dissolved in anhydrous methanol, and 6.2 mL of freshly prepared 1M sodium methoxide solution was added. After the completion of the reaction was monitored by thin-layer chromatography, it was filtered, concentrated, and separated by reverse-phase column chromatography. After elution,...
Embodiment 2
[0020] The method of this embodiment is the same as in Example 1, except that the charging amount of Compound II in Reaction 1 becomes 12.0g, and the final yield of Reaction 1 is 56.2%.
Embodiment 3
[0022] The method of this example is the same as that of Example 1, except that the amount of compound II in reaction 1 is changed to 32.0 g, and the final yield of reaction 1 is 52.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com