Preparation and application of highly specific amantadine artificial antigen
A technology of amantadine and artificial antigen, which is applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve the problems of expensive instruments, time-consuming detection, failure to meet the requirements of fast and accurate, and achieve specificity High performance and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
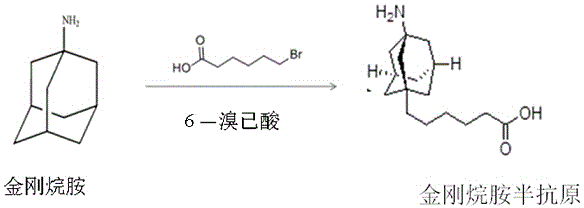
[0032] Example 1 Preparation of a small molecule compound (i.e. amantadine hapten)
[0033] Amantadine (100mg, 0.66mmol) and 6-bromohexanoic acid (130mg, 0.8mmol) were added to toluene, and the temperature was raised to 130°C under nitrogen protection to reflux for 48h. Concentrate under reduced pressure, add water (10 mL), adjust the pH of the aqueous phase to 2-3 with hydrochloric acid, add ethyl acetate for extraction, wash with saturated NaCl solution, dry and concentrate to obtain the amantadine hapten product.
Embodiment 2
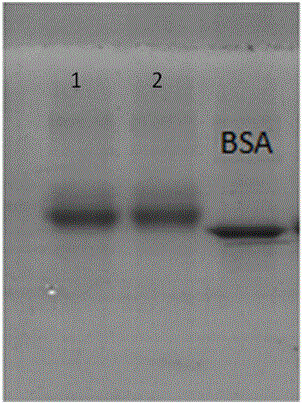
[0034] Example 2 Preparation of amantadine artificial antigen (synthesized immunogen by EDC method)
[0035] Take 10mg of amantadine hapten, add 2mL of ultrapure water to dissolve, then add NHS and EDC respectively (the molar ratio of hapten, NHS, and EDC is 1: 2: 2), mix well at room temperature, and react at room temperature at 25°C 12h. Take 25mg bovine serum albumin (the molar ratio of hapten and bovine serum albumin is 80:1), add 5mL 0.1M pH9.6 carbonate buffer. The activated hapten solution was slowly added dropwise to the protein solution, and reacted at room temperature for 24 hours. The amantadine artificial antigen was obtained by dialysis with PBS buffer solution for 2 days, during which the water was changed 4 times.
Embodiment 3
[0036] Example 3 Preparation of amantadine artificial antigen (synthesis of immunogen by mixed anhydride method)
[0037] Take 9 mg of amantadine hapten and add 2 mL of ultrapure water to dissolve it. At 0°C, add tri-n-butylamine and isobutyl chloroformate (the molar ratio of hapten, tri-n-butylamine, and isobutyl chloroformate is 1: 1.5: 1.5), and react at 0°C for 1 hour. Take 30mg of bovine serum albumin (the molar ratio of hapten to bovine serum albumin is 60:1), add 5mL of 0.1M pH9.6 carbonate buffer, and pre-cool at 0°C for 30min. At 0°C, the activated hapten solution was slowly added dropwise to the protein solution, reacted at 0°C for 1 hour, and then reacted at room temperature for 24 hours. The amantadine artificial antigen was obtained by dialysis with PBS buffer solution for 2 days, during which the water was changed 4 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com