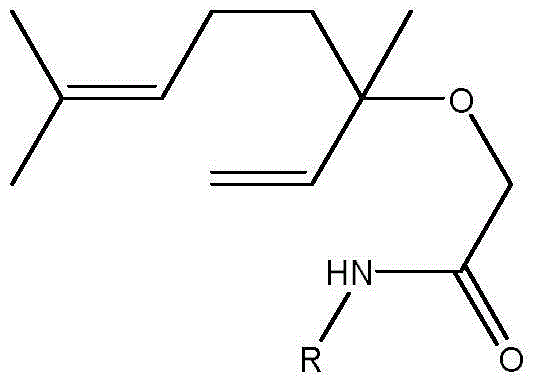
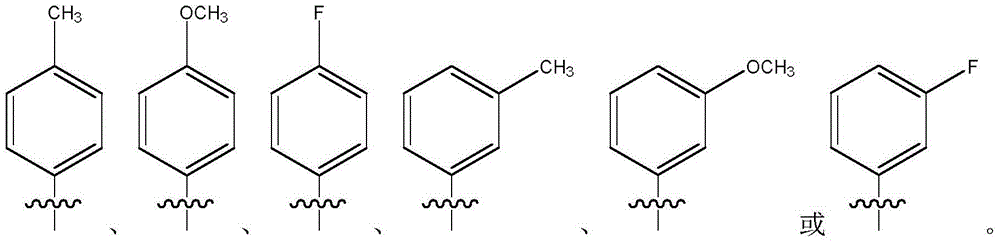
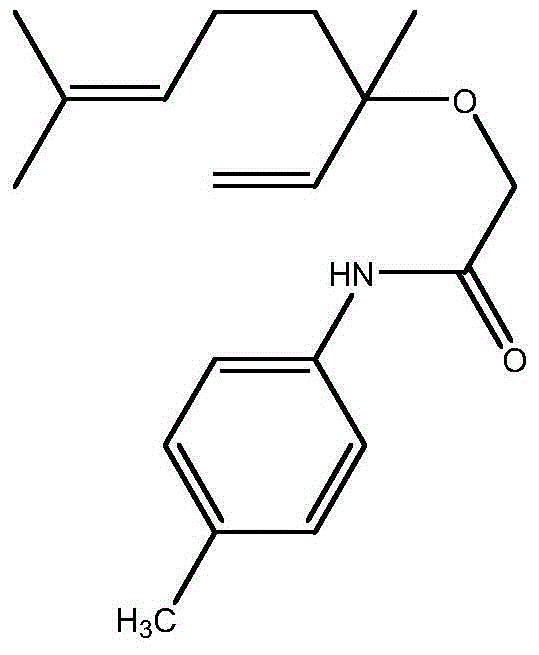
Amide derivatives of linalool, preparation method of amide derivatives and application of amide derivatives in bacteria prevention
A technology of linalool and amides, which is applied in the preparation of carboxylic acid amides, antibacterial drugs, organic compounds, etc., and can solve the problems of not reaching treatment and accelerating the speed of drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 2-(3,7-Dimethyl-1,6-octadien-3-ol)-N-(p-phenylmethyl)acetamide (compound 1)
[0025]
[0026] Step 1: 30.1 g of linalool and 13.8 g of potassium carbonate were added to 100 mL of anhydrous DMF solution, and after stirring, 15.2 g of methyl bromoacetate in 10 mL of DMF solution was added dropwise at 0°C. The reaction solution was reacted at 60° C. for 8 hours, then added with 300 mL of water, extracted three times with ethyl acetate, and dried to obtain a crude product. The crude product was crystallized from ethanol to obtain methyl 3,7-dimethyl-1,6-octadien-3-ol acetate.
[0027] Step 2: Add 20 g of methyl 3,7-dimethyl-1,6-octadien-3-ol acetate into 100 mL of 10% NaOH aqueous solution, heat to reflux for 2 h, extract with ethyl acetate, and keep the water phase. Dilute HCl solution was added dropwise to the aqueous phase until the precipitation did not increase to obtain 3,7-dimethyl-1,6-octadien-3-ol acetic acid.
[0028] Step 3: 21.2 g of compound C, 13.5 g of H...
Embodiment 2
[0031] 2-(3,7-Dimethyl-1,6-octadien-3-ol)-N-(m-benzyl)acetamide (compound 2)
[0032]
[0033] The specific steps are the same as in Example 1, except that p-toluidine in Step 3 of Example 1 is replaced by m-toluidine.
[0034] The obtained compound was pale yellow. 1 H NMR(DMSO,300MHz)δ:9.96(s,1H,NH),7.25(m,1H),6.98(d,1H,J=2.1Hz),6.95(m,1H),6.90(dd,1H, J 1 =8.3Hz J 2 =2.1Hz), 5.99(dd,1H,J 1 =15.8Hz J 2 =10.8Hz),5.4(m,1H),5.17(dd,1H,J 1 =15.8Hz J 2 =1.7Hz), 5.07(dd,1H,J 1 =10.8Hz J 2 =1.7Hz),2.26(s,3H),2.22(m,4H),1.27(m,6H),1.22(m,3H).MS(ESI):302(C 19 h 28 NO 2 ,[M+H]+).Anal.Calcd for C 19 h 27 NO 2 :C,75.71;H,9.03;O,10.62Found:C,75.73;H,3.55;O,10.37
Embodiment 3
[0036] 2-(3,7-Dimethyl-1,6-octadien-3-ol)-N-(p-phenylmethoxy)acetamide (Compound 3)
[0037]
[0038] The specific steps are the same as in Example 1, except that the p-toluidine in step 3 of Example 1 is replaced by p-methoxyaniline.
[0039] The obtained compound was pale yellow. 1H NMR (DMSO, 300MHz) δ: 9.96 (s, 1H, NH), 7.06 (d, 1H, J = 2.1Hz), 6.96 (d, 1H, J = 8.2Hz), 6.83 (dd, 1H, J 1 =8.5Hz J 2 =2.3Hz), 6.72(d,1H,J=8.5Hz),5.94(dd,1H,J 1 =15.8Hz J 2 =10.8Hz),5.53(m,1H),5.15(dd,1H,J 1 =15.8Hz J 2 =1.7Hz), 5.04(dd,1H,J 1 =10.8Hz J 2 =1.7Hz),3.86(s,3H),2.23(m,4H),1.28(m,6H),1.22(m,3H).MS(ESI):318(C 19 h 28 NO 3 ,[M+H]+).Anal.Calcd for C 19 h 27 NO 3 :C,71.89;H,8.57;O,15.12Found:C,71.81;H,8.55;O,15.12
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com