Preparation method and application of cucurbitacin c and its analogs
A technology of cucurbitacin and its analogues, which is applied in the field of natural medicinal chemistry and can solve the problems of not introducing the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Extraction of Cucurbitacin C, Deacetylcucurbitacin C, Dihydrocucurbitacin C, and Dihydrodeacetylcucurbitacin C
[0037] 1. Extraction and separation
[0038] 70kg of cucumber leaves were chopped and extracted for 1 hour with 95% ethanol under reflux, and extracted 3 times in total. The solvent was concentrated under reduced pressure at 90°C to obtain 780 g of solid extract. Suspend 700 g of the solid extract in water, extract 8 times with petroleum ether, remove the petroleum ether layer, and collect the water layer. Centrifuge the water layer, collect the filtrate, and use D101 macroporous resin column for chromatographic separation. The length of the column bed is 150cm, and the inner diameter of the column is 20cm. According to the flow rate of 40ml / min, water and ethanol of different concentrations are eluted in sequence: the water elution is Site 1, 30% ethanol eluted as site 2, 80% ethanol eluted as site 3, 95% ethanol eluted as site 4, each site was e...
Embodiment 2
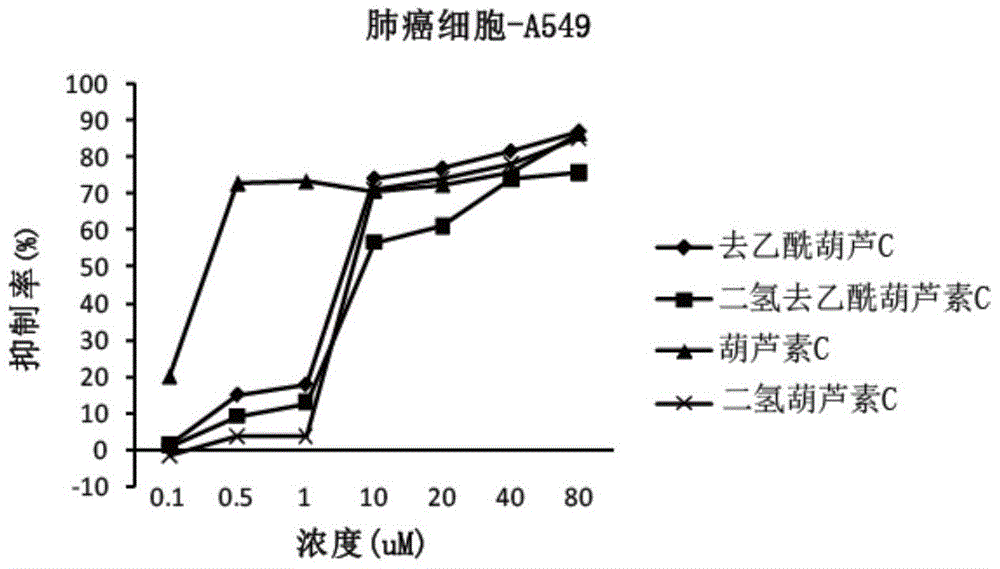
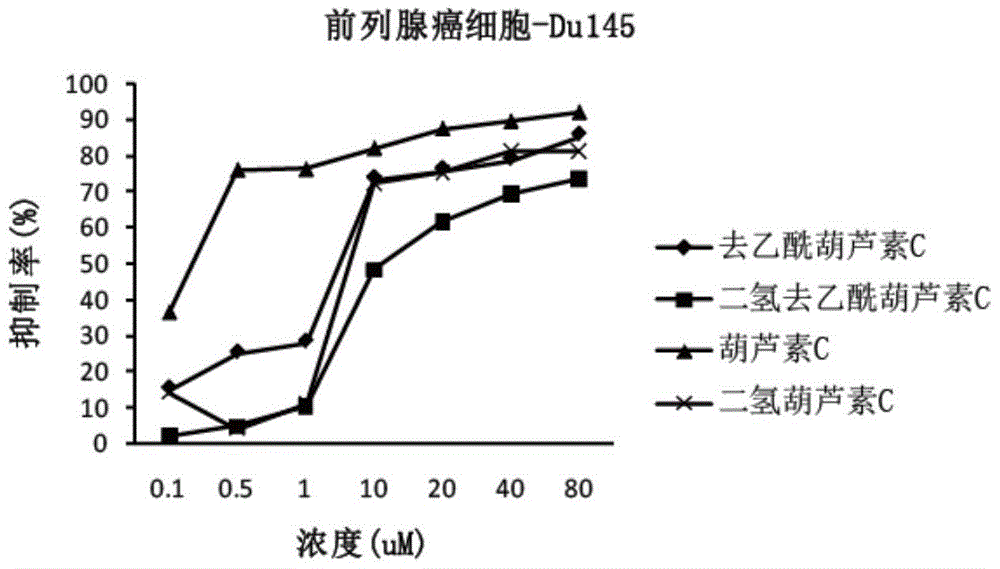
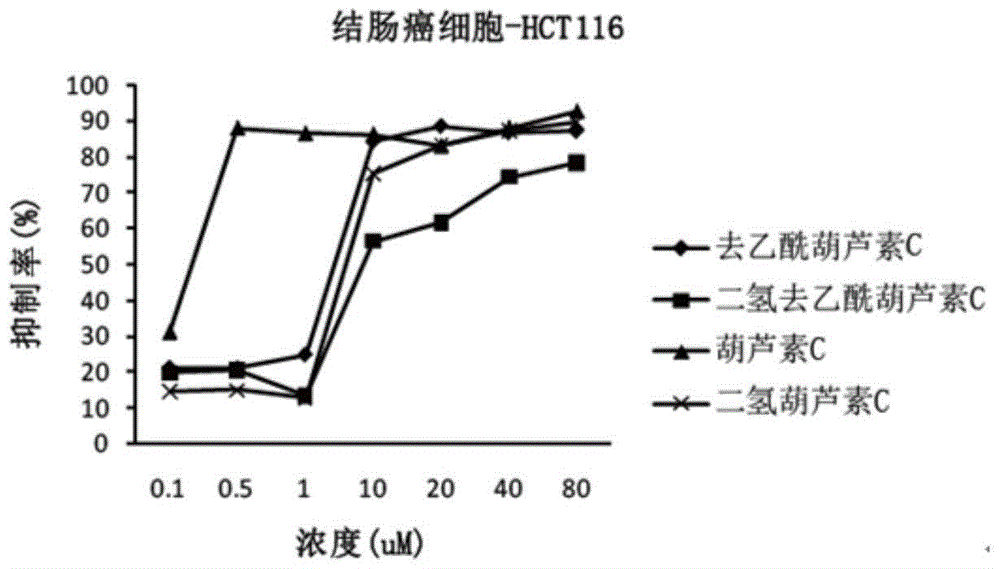
[0069] Example 2 Anticancer Activity Cell Experiment of Cucurbitacin C and Its Analogs
[0070] 1. Experimental cells
[0071] Human lung cancer cell line A549, human prostate cancer cell line Du145, and human colon cancer cell line HCT116.
[0072] The above cell lines were respectively cultured in RPMI1640 complete culture medium supplemented with 10% inactivated newborn calf serum. Add 100IU / mL penicillin, 100μg / mL streptomycin and 10mM HEPES to the culture medium, and place at 37°C, 5% CO 2 cultured in an incubator. The cells used in the experiment were all in the logarithmic growth phase.
[0073] 2. Experimental drugs
[0074] Four kinds of cucurbitacins prepared in Example 1: cucurbitacin C, deacetylcucurbitacin C, dihydrocucurbitacin C, and dihydrodeacetylcucurbitacin C; 201102) as a positive control drug. The drug concentration of 0.1 μM-80 μM is prepared in the culture medium.
[0075] 3. Determination of anticancer activity
[0076] Determination by MTT meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com