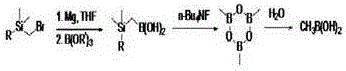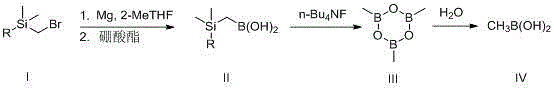A kind of method for preparing methyl boric acid
A technology of methyl boric acid and boric acid ester, which is applied in the field of preparation of methyl boric acid, can solve the problems of production safety hazards, difficult extraction, waste of solvents, etc., and achieve the effects of simplified operation, easy acquisition, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of (trimethylsilyl)methylboronic acid: Under nitrogen protection, add metal magnesium (0.33 moles) and a few small grains of iodine to a reaction flask equipped with a condenser tube and a constant pressure feeding funnel, and add trimethylsilyl dropwise under stirring. 30 ml of a mixed solution of methyl bromide (0.3 mol) dissolved in 250 ml of 2-methyltetrahydrofuran. Heat to above 40°C, and slowly add the remaining solution dropwise after the reaction is initiated. The temperature was raised to reflux for 3 hours, and the temperature was lowered to room temperature. In another reaction flask, under the protection of nitrogen, add trimethyl borate (0.35 mol) and 80 ml of 2-methyltetrahydrofuran, stir evenly, cool down to -20°C, and slowly add the Grignard reagent prepared above, 2 After adding -3 hours, keep warm and continue to react for 2-2.5 hours. When it is confirmed that the reaction does not change, raise the temperature to 0°C and add 10% hydrochlor...
Embodiment 2
[0028] Synthesis of (dimethyl tert-butylsilyl) methylboronic acid: under nitrogen protection, add metal magnesium (0.40 moles) and a few small grains of iodine to a reaction flask equipped with a condenser tube and a constant-pressure feeding funnel, and add two drops of iodine under stirring. 30 ml of a mixed solution of methyl tert-butylsilyl bromide (0.4 mol) dissolved in 250 ml of 2-methyltetrahydrofuran. Heat to above 50°C, and slowly add the remaining solution dropwise after the reaction is initiated. The temperature was raised to reflux for 3 hours, and the temperature was lowered to room temperature. In another reaction flask, under the protection of nitrogen, add trimethyl borate (0.33 mol) and 100 ml of 2-methyltetrahydrofuran, stir evenly, cool down to -20°C, and slowly add the Grignard reagent prepared above, 2 After adding -3 hours, keep warm and continue to react for 2-2.5 hours. When it is confirmed that the reaction does not change, raise the temperature to 0°...
Embodiment 3
[0034] Synthesis of (trimethylsilyl)methylboronic acid: Under nitrogen protection, add metal magnesium (0.33 moles) and a few small grains of iodine to a reaction flask equipped with a condenser tube and a constant pressure feeding funnel, and add trimethylsilyl dropwise under stirring. 30 ml of a mixed solution of methyl bromide (0.3 mol) dissolved in 350 ml of 2-methyltetrahydrofuran. Heat to above 40°C, and slowly add the remaining solution dropwise after the reaction is initiated. The temperature was raised to reflux for 3 hours, and the temperature was lowered to room temperature. In another reaction flask, under the protection of nitrogen, add triisopropyl borate (0.33 moles) and 180 ml of 2-methyltetrahydrofuran, stir evenly, cool down to -10°C, and slowly add the Grignard reagent prepared above, After 2-3 hours of addition, keep warm and continue to react for 2-2.5 hours. When it is confirmed that the reaction does not change, raise the temperature to 0°C, add 10% hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com