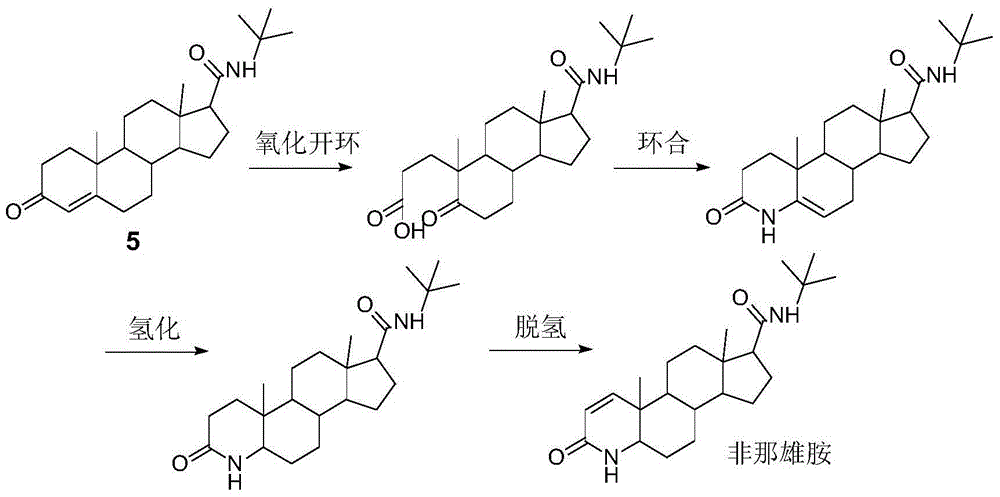
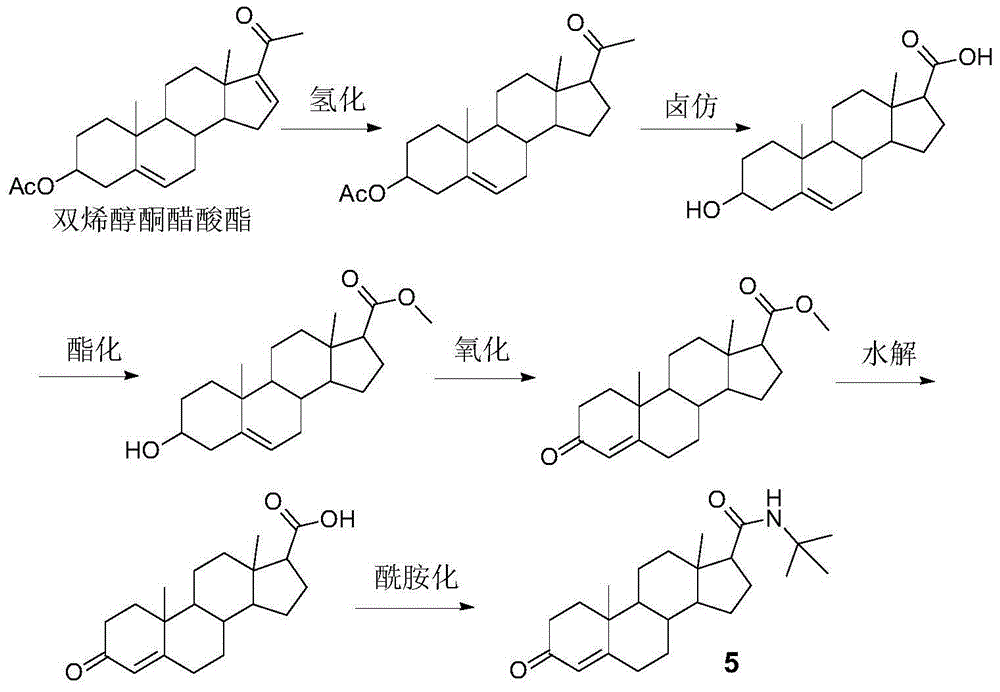
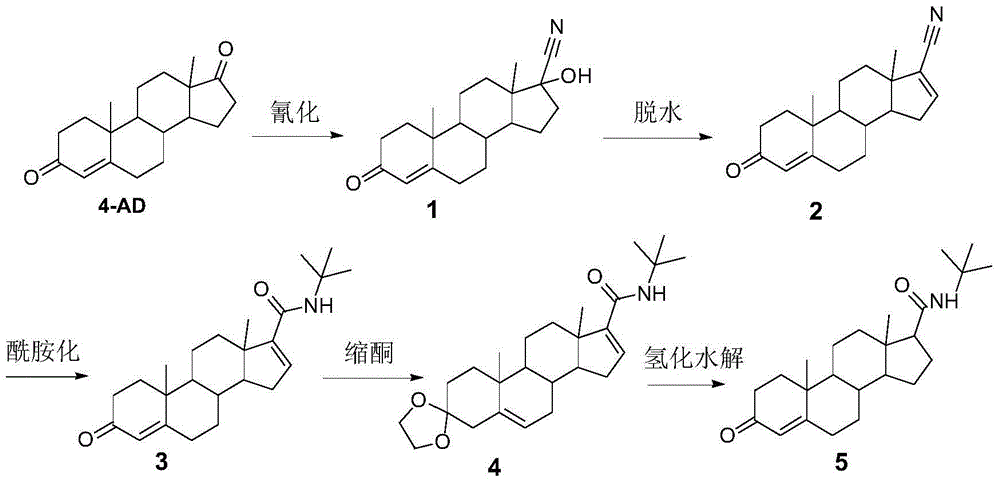
Method for preparing finasteride intermediate
A finasteride and intermediate technology, applied in the field of chemical drug synthesis, can solve the problems of increased raw material cost and environmental protection, achieve high production application and economic value, easy control, increase solubility and reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Put 30 g of methanol, 10 g of acetone cyanohydrin and 10 g of raw material 4AD into the reaction vessel, then add potassium carbonate aqueous solution (1.0 g of potassium carbonate + 20 g of water), and react at 40-45° C. for about 20 hours. After the reaction, the temperature is lowered to 0~10℃, the pH is adjusted to 4~5 with 10% dilute hydrochloric acid, washed into cold water for crystallization, filtered and washed with water until the filter cake is neutral to obtain the crude product, which is refined with acetone water to obtain 17-hydroxycyanide- 4-Androsten-3-one (Compound 1), dried to obtain 10.4g. The HPLC content is 96.4%, with 3.2% isomers.
[0040]
[0041] 10g of compound 1, 70g of pyridine, and 15g of phosphorus oxychloride are added to the reaction vessel, and the temperature is raised to 100-110°C for 0.5 hour. After the reaction, the temperature is lowered to below 40°C, quickly washed into ice water for crystallization, filtered and washed wi...
Embodiment 2
[0049] Put 120 g of methanol, 32 g of acetone cyanohydrin and 40 g of raw material 4AD into the reaction vessel, and then add potassium carbonate aqueous solution (4.8 g of potassium carbonate + 80 g of water), and react at 40-45° C. for about 24 hours. After the reaction, the temperature is lowered to 0~10℃, the pH is adjusted to 4~5 with 10% dilute hydrochloric acid, washed into cold water for crystallization, filtered and washed with water until the filter cake is neutral to obtain the crude product, which is refined with acetone water to obtain 17-hydroxycyanide- 4-Androsten-3-one (Compound 1), dried to obtain 41.5g. HPLC content is 96.8%, containing 2.9% isomer.
[0050] 40 g of compound 1, 160 g of pyridine, and 32 g of phosphorus oxychloride were added to the reaction vessel, and the temperature was raised to 100-110° C. to react for 1 hour. After the reaction, the temperature is lowered to below 40°C, quickly washed into ice water for crystallization, filtered and washed...
Embodiment 3
[0055] Put 500g methanol, 225g acetone cyanohydrin and 150g raw material 4AD into the reaction vessel, then add potassium carbonate aqueous solution (9g potassium carbonate+300g water), keep the temperature at 40-45°C for about 16 hours. After the reaction, the temperature is lowered to 0~10℃, the pH is adjusted to 4~5 with 10% dilute hydrochloric acid, washed into cold water for crystallization, filtered and washed with water until the filter cake is neutral to obtain the crude product, which is refined with acetone water to obtain 17-hydroxycyanide- 4-Androsten-3-one (Compound 1) was dried to obtain 159g. The HPLC content is 96.1%, with 3.7% isomer.
[0056] 150g of compound 1, 750g of pyridine, and 150g of phosphorus oxychloride were added to the reaction vessel, and the temperature was raised to 100-110°C for 0.75 hours. After the reaction, the temperature is lowered to below 40°C, quickly washed into ice water for crystallization, filtered and washed with water until the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com