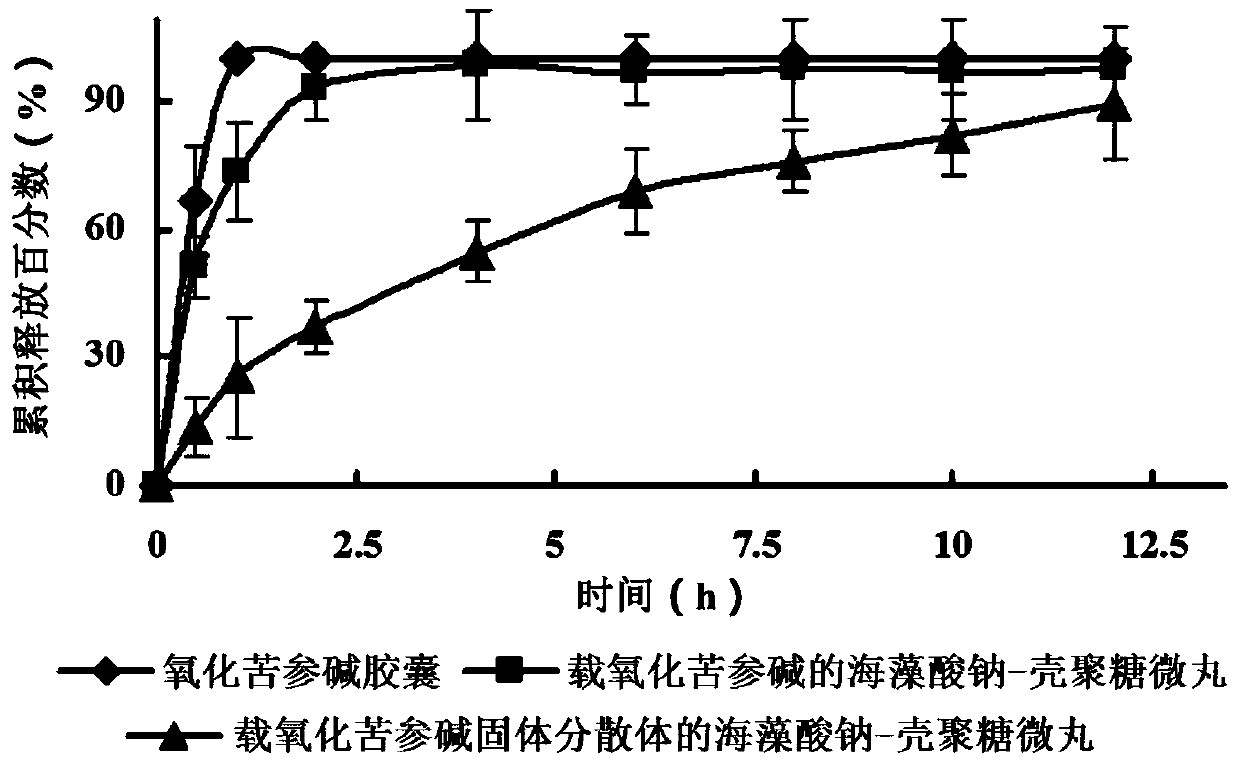
Gastric floating sustained-release pellets loaded with oxymatrine solid dispersion and preparation method thereof
A ginseng solid, slow-release pellet technology, applied in the directions of digestive system, antiviral agents, allergic diseases, etc., can solve the problem that the sustained-release, long-acting, pharmacological activity and therapeutic effect of oxymatrine cannot be achieved, Oxymatrine reduces the bioavailability and other problems, and achieves the effects of good sustained-release drug release characteristics, good flotation performance, and good in vivo gastric positioning.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Taking the sodium alginate-chitosan gastric floating sustained-release pellets loaded with oxymatrine solid dispersion of the present invention as an example, the types of materials used and their proportions are as follows:
[0032] Oxymatrine solid dispersion:
[0033] Material name
Proportion
Oxymatrine: Ethylcellulose
1:1
[0034] Sodium alginate matrix layer with cavities:
[0035] Material name
Dosage
Oxymatrine Solid Dispersion
0.25g
0.2g
0.4g
0.2g
water
10mL
[0036] Slow-release material wrap:
[0037] Material name
Dosage
0.15g
36% acetic acid
1.6mL
water
10mL
[0038] The gastric floating sustained-release pellets described in this example are prepared by the following method:
[0039] 1) Preparation of oxymatrine solid dispersion: dissolve oxymatrine ...
Embodiment 2
[0044] Taking the sodium alginate-chitosan gastric floating sustained-release pellets loaded with oxymatrine solid dispersion of the present invention as an example, the types of materials used and their proportions are as follows:
[0045] Oxymatrine solid dispersion:
[0046] Material name
Proportion
Oxymatrine: Ethylcellulose
1:1
[0047] Sodium alginate matrix layer with cavities:
[0048] Material name
Dosage
Oxymatrine Solid Dispersion
0.5g
0.5g
0.5g
0.5g
water
10mL
[0049] Slow-release material wrap:
[0050] Material name
Dosage
0.2g
36% acetic acid
3.2mL
water
10mL
[0051] The gastric floating sustained-release pellets described in this example are prepared by the following method:
[0052] 1) Preparation of oxymatrine solid dispersion: dissolve oxymatrine and ...
Embodiment 3
[0057] Taking the sodium alginate-chitosan gastric floating sustained-release pellets loaded with oxymatrine solid dispersion of the present invention as an example, the types of materials used and their proportions are as follows:
[0058] Oxymatrine solid dispersion:
[0059] Material name
Proportion
Oxymatrine: Ethylcellulose
1:3
[0060] Sodium alginate matrix layer with cavities:
[0061] Material name
Dosage
Oxymatrine Solid Dispersion
0.1g
sodium alginate
0.1g
calcium chloride
0.4g
0.1g
water
10mL
[0062] Slow-release material wrap:
[0063] Material name
Dosage
0.05g
36% acetic acid
0.6mL
[0064] water
10mL
[0065] The gastric floating sustained-release pellets described in this example are prepared by the following method:
[0066] 1) Preparation of oxymatrine solid dispersion: diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| floating rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com