In-situ preparation method of zinc phthalocyanine/carbon nanotube composite catalyst based on solvothermal method
A carbon nanotube composite and carbon nanotube technology, which is applied in the field of material chemistry, can solve the problems of long reaction process time, troublesome post-processing, consumption of raw materials, etc., and achieve the effects of effective degradation, short reaction time, and easy post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
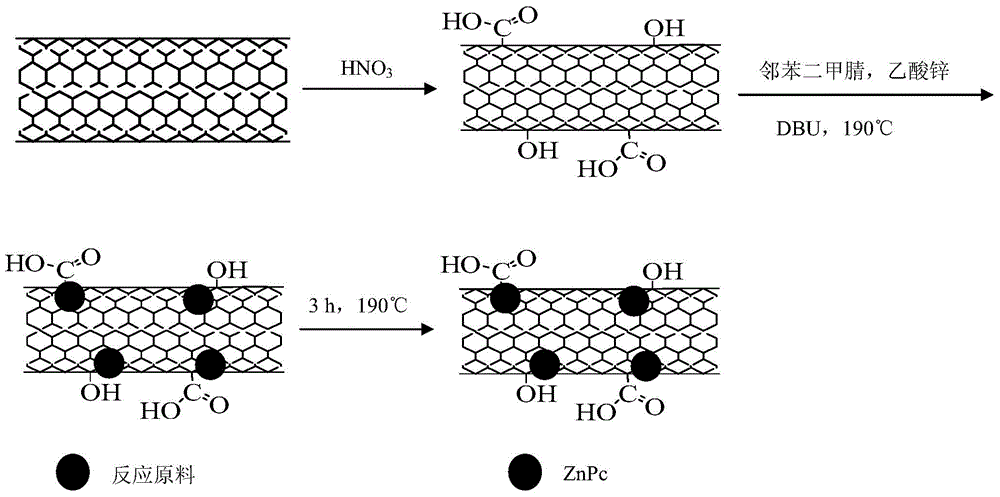
[0020] (1) Pretreatment of carbon nanotubes
[0021] Weigh 0.3029g of multi-walled carbon nanotubes, add 150mL of 65% concentrated nitric acid, heat and stir at 120°C for 9h, cool after the reaction, wash with water until neutral, and dry to obtain purified carbon nanotubes.
[0022] (2) Preparation of zinc phthalocyanine / carbon nanotube composites
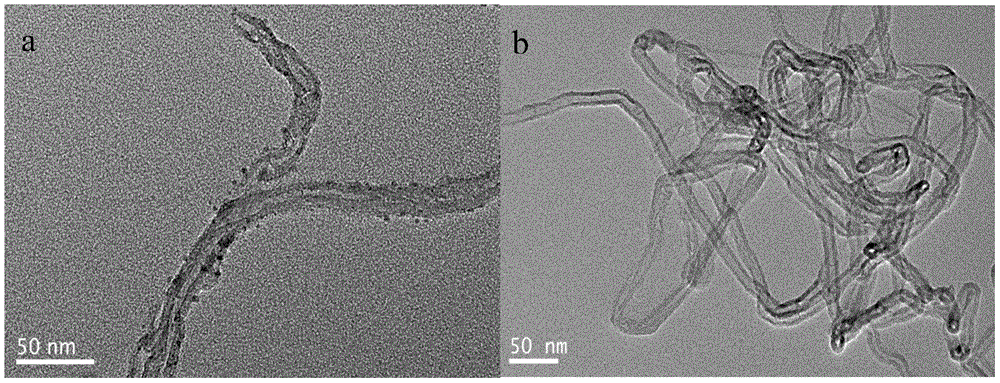
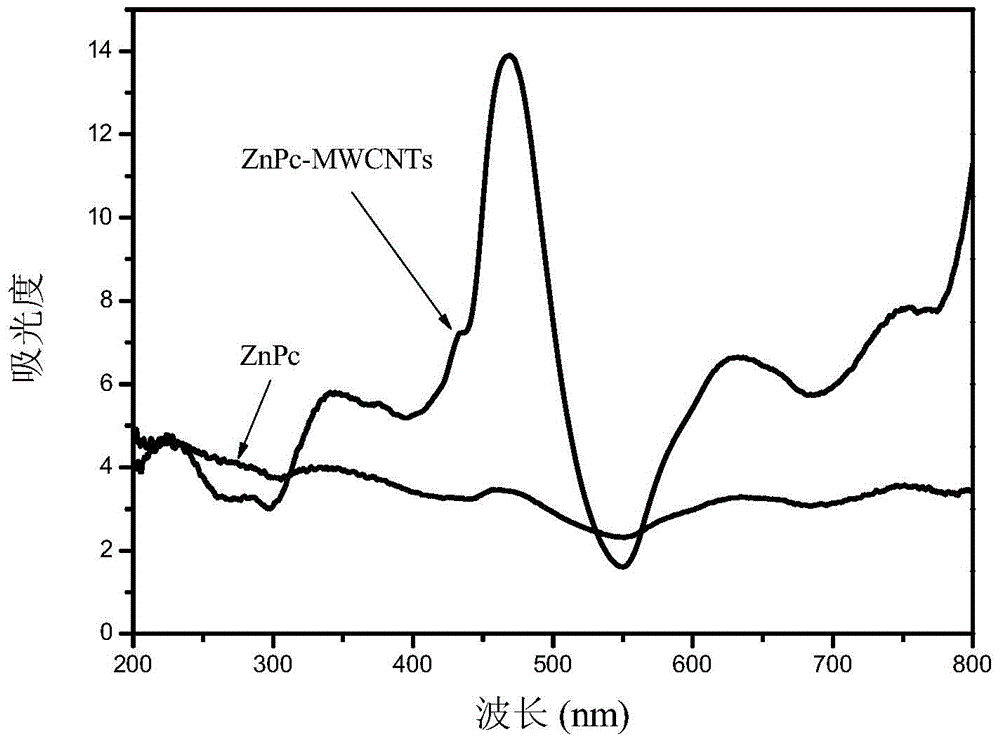
[0023] Weigh 1.5mmol of zinc acetate dihydrate, 6mmol of phthalonitrile, 0.25g of carbon nanotubes and 0.2mL of 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU) , add 50ml of absolute ethanol as a solvent, stir for 30 minutes and then pour it into the reaction kettle. After the reaction kettle is sealed, carry out solvothermal reaction at 190°C for 2 hours. After the reaction, stop heating and cool to room temperature naturally. The sample prepared above was taken out, and the remaining reactants adsorbed on the surface of the zinc phthalocyanine / carbon nanotubes were washed repeatedly with purified water and absolute ethanol, and then p...
Embodiment 2
[0025] (1) Pretreatment of carbon nanotubes
[0026] Weigh 0.5010g of multi-walled carbon nanotubes, add 150mL of 65% concentrated nitric acid, heat and stir at 120°C for 9h, cool after the reaction, wash with water until neutral, and dry to obtain purified carbon nanotubes.
[0027] (2) Preparation of zinc phthalocyanine / carbon nanotube composites
[0028] Weigh 1.5mmol of zinc acetate dihydrate, 6mmol of phthalonitrile, 0.4g of carbon nanotubes and 0.5mL of 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU) , add 50ml of absolute ethanol as a solvent, stir for 30 minutes and then pour it into the reaction kettle. After the reaction kettle is sealed, carry out solvothermal reaction at 190°C for 4 hours. After the reaction, stop heating and cool to room temperature naturally. The sample prepared above was taken out, and the remaining reactants adsorbed on the surface of the zinc phthalocyanine / carbon nanotubes were washed repeatedly with purified water and absolute ethanol, and then pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com