Method for preparing glucosyl group 5-hydroxymethy furfural through ionic liquid and three chlorizated salt
A technology of hydroxymethylfurfural and ionic liquids, which is applied in the field of biomass sugar resource utilization, can solve the problems of unfavorable industrial production, high microwave radiation power, and expensive ionic liquids, so as to avoid the difficulty of solvent recovery, reduce pollution, Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
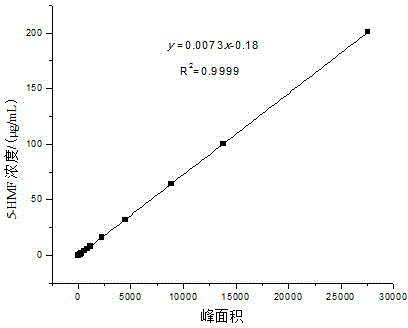
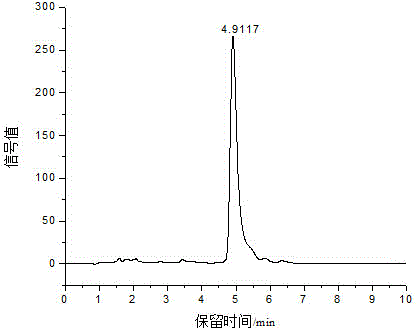
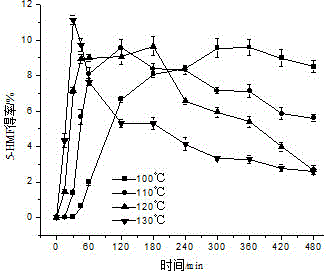
[0046] Add glucose (100 mg) and 1-butyl-3-methylimidazolium chloride ([BMIM]Cl, 1 g) to 5 mL ampoules, and ultrasonically assisted dissolution: at ultrasonic frequency and power of 25 kHz and 600 W, respectively, at 28 kHz and Under the conditions of 600W, 40kHz and 600W, the three-frequency simultaneous ultrasonic treatment was performed for 15min each, and the temperature was 25-30°C. The sonicated solution was placed in a water bath at 50°C for preheating for 15min, and FeCl was added. 3 ·6H 2 O (10mg), carry out nitrogen saturation treatment for 5min, and seal with alcohol blowtorch. Under the heating conditions of 100 ℃, 110 ℃, 120 ℃ and 130 ℃ oil bath, respectively, heat for 8 hours. For the first 1 hour, take a sample every 15 minutes, and then take a sample every 1 hour for the next 7 hours. Three parallels were performed for each sample. With the increase of the reaction temperature and the prolongation of the reaction time, the yield of 5-HMF increased continuously...
Embodiment 2
[0048] Add glucose (100 mg) and 1-butyl-3-methylimidazolium chloride ([BMIM]Cl, 1 g) to 5 mL ampoules, ultrasonic-assisted dissolution: at ultrasonic frequency and power of 25 kHz and 600 W, respectively, at 28 kHz and Under the conditions of 600W, 40kHz and 600W, the three-frequency simultaneous ultrasonic treatment was performed for 15min each, and the temperature was 25-30°C. The solution after ultrasonication was placed in a water bath at 50°C for preheating for 15min, and AlCl was added. 3 (10mg), irrigated with nitrogen for 5 minutes, and sealed with an alcohol torch. Under the heating conditions of 100 ℃, 110 ℃, 120 ℃ and 130 ℃ oil bath, respectively, heat for 8 hours. For the first 1 hour, take a sample every 15 minutes, and then take a sample every 1 hour for the next 7 hours. Three parallels were performed for each sample. The highest yield of 5-HMF reached was 27.68 ± 1.26% at 130 °C for 30 min, and it also showed a downward trend after that, for the same reasons a...
Embodiment 3
[0050] Add glucose (100 mg) and 1-butyl-3-methylimidazolium chloride ([BMIM]Cl, 1 g) to 5 mL ampoules, ultrasonic-assisted dissolution: at ultrasonic frequency and power of 25 kHz and 600 W, respectively, at 28 kHz and Under the conditions of 600W, 40kHz and 600W, the three-frequency simultaneous ultrasonic treatment was performed for 15min each, and the temperature was 25-30℃. The solution after ultrasonication was placed in a water bath at 50℃ for preheating for 15min, and CrCl was added. 3 ·6H 2 O (10mg), saturated with nitrogen for 5min, and sealed with an alcohol torch. Under the conditions of oil bath heating at 100°C, 110°C, 120°C, and 130°C, respectively, heat for 8h, take samples every 15min for the first 1 hour, and take samples every 1h for the last 7 hours. Three parallels were performed for each sample. At 120 °C, the yield of 5-HMF reached 31.66 ± 2.09% after 60 min of reaction, and then showed a downward trend for the same reasons as above. Distill the reacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com