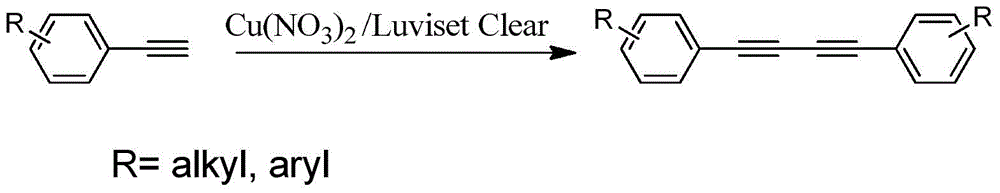
Novel method for catalytically synthesizing 1,3-diyne compound with simple, highly efficient and reusable copper catalytic system
A synthesis method and technology of catalytic system, applied in the directions of hydrocarbons, hydrocarbons, chemical instruments and methods, etc., can solve the problems of environmental pollution, corrosion, speeding up the reactor, etc. mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0017] Add 1mmol phenylacetylene, Cu(NO 3 ) 2 0.01mmol, 50mg Luviset Clear and 2mL toluene were reacted at 100°C for 4h. After the reaction, the mixture was filtered, concentrated, and separated by column chromatography to obtain 1,4-diphenyl-1,3-butadiyne with a yield of 97%.
preparation example 2
[0019] Add 1mmol phenylacetylene, Cu(NO 3 ) 2 0.10mmol, 150mg Luviset Clear and 2mL toluene, the reaction was carried out at 100°C for 4h. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography to obtain 1,4-diphenyl-1,3-butadiyne with a yield of 31%.
preparation example 3
[0021] Add 1mmol phenylacetylene, Cu(OAc) to a 10mL reaction tube 2 0.02mmol, 86mg Luviset Clear and 2mL 1,4-dioxane, the reaction was carried out at 25°C for 4h. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography to obtain 1,4-diphenyl-1,3-butadiyne with a yield of 48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com