Method for preparing epsilon-caprolactone from cyclohexanone by in-situ oxidation
An in-situ oxidation and cyclohexanone technology, applied in organic chemistry and other directions, can solve the problems of high material consumption and energy consumption, many processes, and high requirements for safety measures, and achieve production process safety, shorten the process flow, and require low equipment safety factors. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
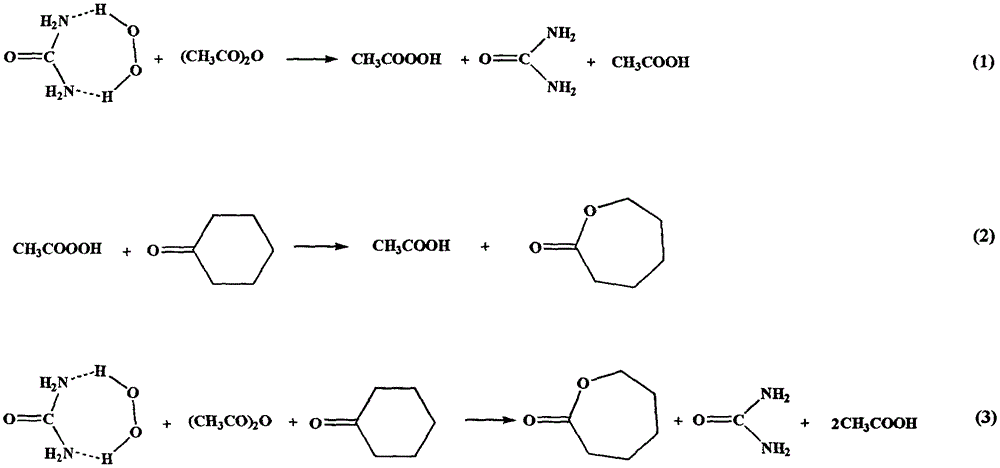
[0021] Weigh 112g of percarbamide with an active oxygen content of 14.3% and add it to a glass reactor with a stirring device, add 96g of ethyl acetate, then weigh 49g of cyclohexanone and add it to the reactor, start the stirrer to evenly disperse percarbamide in A mixture of ethyl acetate and cyclohexanone. Then the reaction kettle was heated until the ethyl acetate was slightly refluxed and 110 g of acetic anhydride was injected through a constant pressure dropping funnel. During the process of adding acetic anhydride, percarbamide was gradually dissolved, and the reaction mixture was gradually transparent. After the addition of acetic anhydride, the temperature of the reaction solution was automatically lowered, during which a white solid formed during the reaction gradually precipitated, and the reaction was maintained at 70°C for 3 hours. After the reaction is completed, cool the reaction liquid to 0°C, and filter the precipitated white precipitate with a sand core funne...
Embodiment 2
[0023] Weigh 97g of percarbamide with an active oxygen content of 16.5% and add it to a glass reactor with a stirring device, add 128g of ethyl acetate, then weigh 74g of cyclohexanone and add it to the reactor, start the stirrer to evenly disperse percarbamide in A mixture of ethyl acetate and cyclohexanone. Then the reaction kettle was heated until the ethyl acetate was slightly refluxed and 150 g of acetic anhydride was injected through a peristaltic pump. During the process of adding acetic anhydride, percarbamide was gradually dissolved, and the reaction mixture was gradually transparent. After the addition of acetic anhydride, the temperature of the reaction solution was automatically lowered, during which a white solid formed during the reaction gradually precipitated, and the reaction was maintained at 50°C for 4 hours. After the reaction, the reaction solution was cooled to 3°C, and the precipitated white precipitate was filtered with a sand core funnel while it was c...
Embodiment 3
[0025] Weigh 102g of percarbamide with an active oxygen content of 15.7% and add it to a glass reactor with a stirring device, add 135g of ethyl acetate, then weigh 98g of cyclohexanone and add it to the reactor, start the stirrer to evenly disperse percarbamide in A mixture of ethyl acetate and cyclohexanone. Then heat the reactor until the ethyl acetate is slightly refluxed and inject 200g of acetic anhydride through a constant flow pump. During the process of adding the acetic anhydride, the percarbamide is gradually dissolved, and the reaction mixture is gradually transparent. After the addition of acetic anhydride, the temperature of the reaction solution was automatically lowered, during which a white solid formed during the reaction gradually precipitated, and the reaction was maintained at 30°C for 5 hours. After the reaction is completed, cool the reaction liquid to 5°C, and filter the precipitated white precipitate with a sand core funnel while it is cold. The colorl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com