Substituted tetrahydroquinoline derivative, hydrolysis product thereof, synthetic method and application thereof
A technology of hydrolyzed products and derivatives, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of high price, inability to recycle and reuse, etc., and achieve the effect of green reaction, environmental friendliness and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
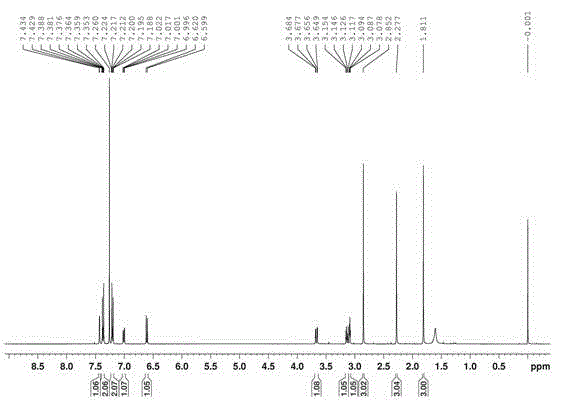
Image
Examples
Embodiment 1
[0028] Preparation of Titanium Dioxide(P25) / NiO Catalyst
[0029] Add 1 g of commercially available titanium dioxide (P25) into 100 mL of nickel acetylacetonate solution (the concentration of the solution is 5×10 -4 mol / L, the proportioning ratio of solvent is ethanol:n-hexane=1:5v / v), stirred at room temperature for 24h, filtered, washed with a small amount of ethanol, then dried at 80°C for 4h, and then placed in a muffle furnace at 500°C Calcined for 1 h to obtain the desired modified titanium dioxide catalyst. Adjust the ratio of nickel in the composite catalyst by repeating the above-mentioned steps, three kinds of catalysts P25 / NiO(1), P25 / NiO(2), P25 / NiO(3) are obtained, wherein the mass percentages of nickel ions are respectively 2.3%, 6.5%, 10.6%.
Embodiment 2
[0031] Visible-light-catalyzed cyclization of N,N-dimethylaniline and 4-chlorophenylmaleimide.
[0032] 0.130g (0.625mmol) of 4-chlorophenylmaleimide, N, N-dimethylaniline 0.151g (1.25mmol), the P25 / NiO (3) (1mol%) catalyst of 0.01g is added pyrex test tube In the test tube, 25ml of acetonitrile was added and dissolved by ultrasound. Then illuminate for 12h under 3W LED blue light. After the reaction was completed, the reaction mixture was concentrated and the product was separated by silica gel column chromatography, using petroleum ether (b.p.60-90°C)-ethyl acetate as eluent for gradient elution. Compound 1 represented by formula III was obtained with a yield of 62%.
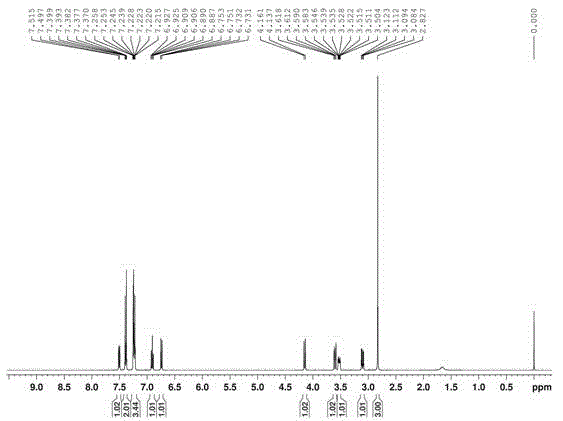
[0033] Compound detection results:
[0034] Melting point: mp 188-190°C; 1 H NMR (CDCl 3 , 400MHz) δ2.83(s, 3H), 3.10(dd, 1H, J=11.4, 4.2Hz), 3.53(ddd, 1H, J=7.2, 4.4, 2.8Hz), 3.60(dd, 1H, J= 11.4, 2.6Hz), 4.15(d, 1H, J=9.6Hz), 6.74(dd, 1H, J=8.2, 0.6Hz), 6.91(td, 1H, J=7.4, 1.1Hz), 7.22-7.26 (m, 3H), 7...
Embodiment 3
[0042] Visible-light-catalyzed cyclization of N,N-dimethylaniline and N-tert-butylmaleimide.
[0043] N-tert-butylmaleimide 0.096g (0.625mmol), N, N-dimethylaniline 0.151g (1.25mmol), 0.01g of P25 / NiO (3) (1mol%) catalyst was added to pyrex In the test tube, add 25ml of acetonitrile to the test tube and dissolve by ultrasonic. Then illuminate for 12h under 3W LED blue light. After the reaction was completed, the reaction mixture was concentrated and the product was separated by silica gel column chromatography, using petroleum ether (b.p.60-90°C)-ethyl acetate as eluent for gradient elution. Compound 2 represented by formula IV was obtained with a yield of 69%.
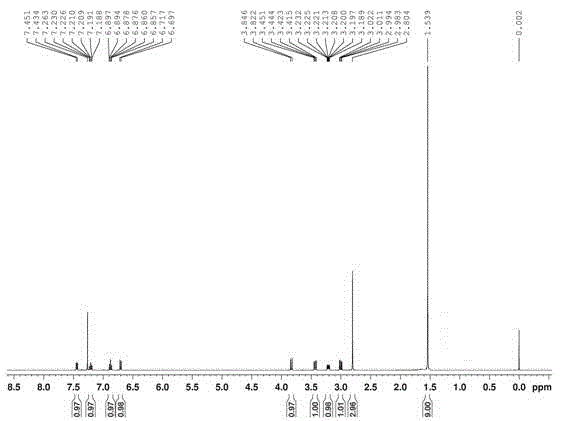
[0044] Compound detection results:
[0045] Melting point: mp 76-78°C; 1 H NMR (CDCl 3 , 400MHz) δ1.54(s, 9H), 2.80(s, 3H), 3.00(dd, 1H, J=11.2, 4.4Hz), 3.21(ddd, 1H, J=7.6, 4.4, 3.0Hz), 3.43 (dd, 1H, J=11.4, 3.0Hz), 3.83(d, 1H, J=9.6Hz), 6.71(d, 1H, J=8.0Hz), 6.88(td, 1H, J=7.6, 1.2Hz) , 7.21 (td, 1H, J=8.2, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com