Amphiphilic illuminant with aggregation induced emission characteristics and applications thereof
An aggregation-induced luminescence and aggregation-induced technology, applied in the field of fluorescent materials, can solve the problems of limited application and achieve the effect of long cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Synthesis of non-ionic amphiphilic luminescent substances P1 / 6, P2 / 6 and P3 / 6 and experimental research on their application
[0082] (1) Synthesis (n, m, o and p respectively represent natural numbers from 2 to 3000)
[0083]
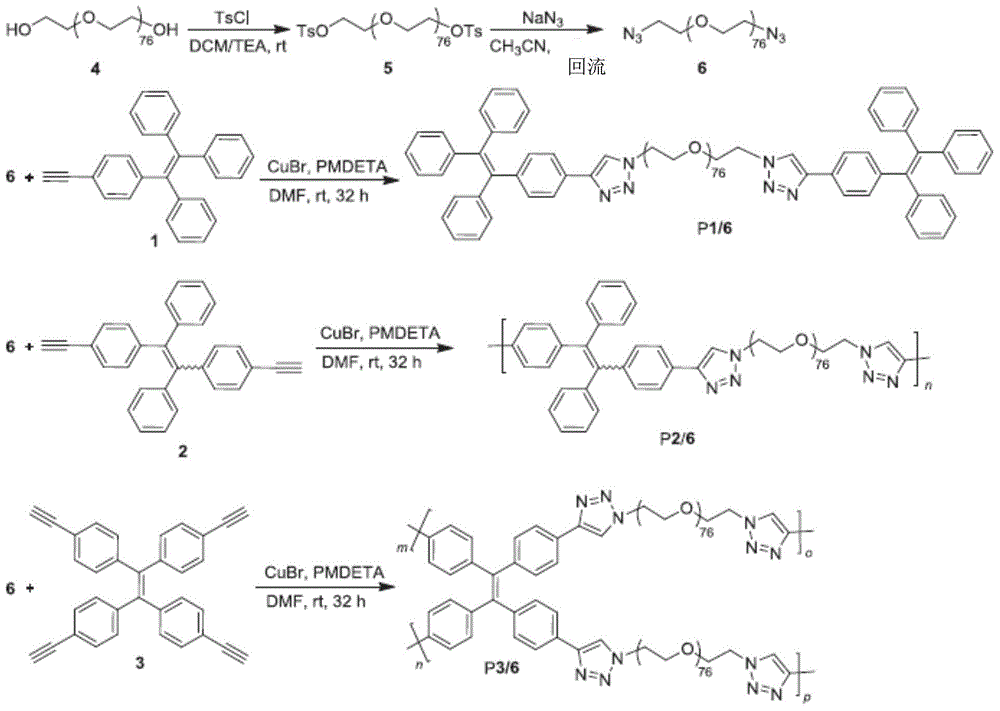
[0084] like figure 1 As shown, the supply ratio of the reagents used in the synthesis of luminescent substance P1 / 6 is as follows, the concentration ratio between the sixth compound 6, the first compound 1, CuBr and PMDETA [6] / [1] / [CuBr] / [PMDETA] is 1 / 4 / 4 / 4, the supply ratio of the reagents for synthesizing luminescent substances P2 / 6 and P3 / 6 is different from that of luminescent substance P1 / 6 in that the sixth compound 6 is different from the second compound 2 and the second compound 6 respectively. The concentration ratio of the three compounds 3 is [6] / [2] / [3]=1 / 1 / 0.5. At room temperature, the sixth compound 6 (1.05 mg, 0.3 mmol) and the first compound 1 (430 mg, 1.2 mmol) was carried out chain reaction, after stirring for ...
Embodiment 2
[0095] Example 2: Synthesis of cationic amphiphilic luminescent material TPE-MEM and experimental research on its application
[0096] (1) synthesis
[0097]
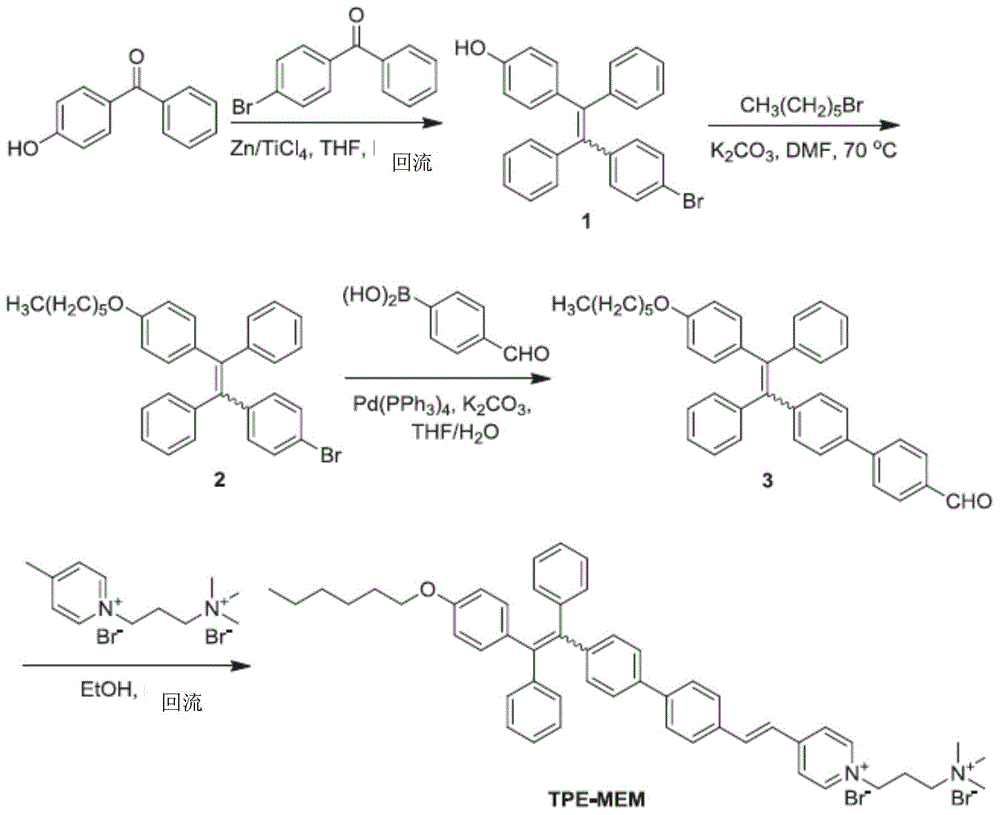
[0098] like figure 2 As shown, under nitrogen atmosphere, 1-(3-trimethylaminopropyl)-4-methylpyridine dibromide (1-(3-trimethylammoniopropyl)-4-methylpyridinium dibromide) (0.5g, 1.4mmol ) and the solution of the third compound 3 (1.5016g, 2.8mmol) were refluxed in absolute ethanol, and three drops of piperidine were added for catalysis. After cooling to room temperature, the solvent was evaporated under reduced pressure and passed through silica gel column chromatography. The residue was purified using a mixed solvent of dichloromethane and methanol (2:1 v / v) as eluent to give a yellow product TPE-MEM (0.72 g, 59%). 1 H NMR (400MHz, methanol-d 4 ,δ):8.948(d,2H,J=6.8Hz),8.213-8.190(m,2H),7.946(6,1H,J=16Hz),7.737-7.717(m,2H),7.576(d,2H ,J=8Hz),7.430-7.331(m,3H),7.061-6.843(m,14H),6.605-6.570(m,2H),4.698(t,2H,J=15...
Embodiment 3
[0113] Example 3: Synthesis of anionic amphiphilic luminescent material TPE-2Gd and experimental research on its application
[0114] (1) Synthesis of TPE-2+
[0115]
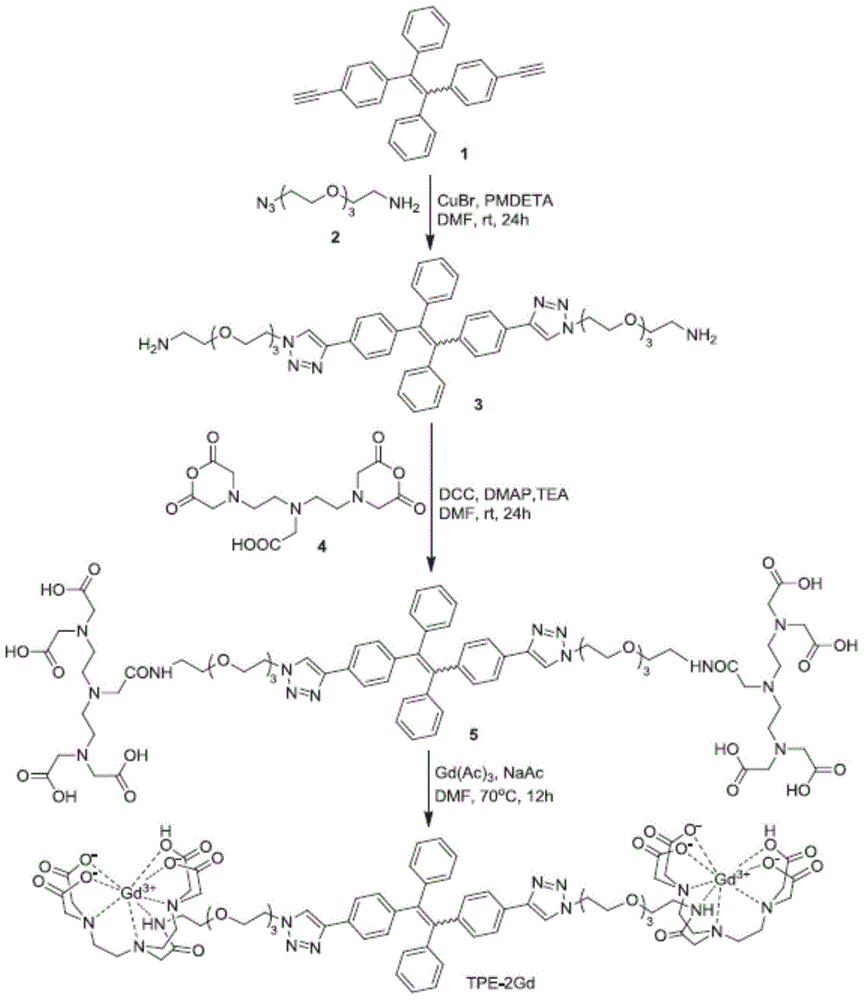
[0116] like image 3As shown, under nitrogen atmosphere, 1-(3-trimethylaminopropyl)-4-methylpyridine dibromide (1-(3-trimethylammoniopropyl)-4-methylpyridinium dibromide) (0.5g, 1.4mmol ) and 4-(1,2,2-triphenylvinyl)benzaldehyde (4-(1,2,2-triphenylvinyl)benzaldehyde) (1.01g, 2.8mmol) were refluxed in anhydrous methanol, Three drops of piperidine were added for catalysis, and after cooling to room temperature, the solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography, using a mixed solvent of dichloromethane and methanol (2:1 v / v) as the eluent. The agent was removed to obtain the yellow product TPE-2+ (0.56g, 57%). 1 H NMR (400MHz, methanol-d 4 ,δ):8.913(d,2H,J=6.8Hz),8.177(d,2H,J=6.8Hz),7.875(d,1H,J=16.0Hz),7.501(d,2H,J=8.4Hz ),7.352(d,1H,J=16.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com