Preparation method for lithium silicate CO2 adsorbent
A technology of CO2 and lithium silicate, applied in the direction of silicate, alkali metal silicate, chemical instruments and methods, etc., can solve the problems of failure to effectively improve lithium silicate adsorbent, prolongation of adsorption-regeneration cycle process, and adsorption rate Low-level problems, to achieve the effect of simple operation, simple preparation process and sufficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
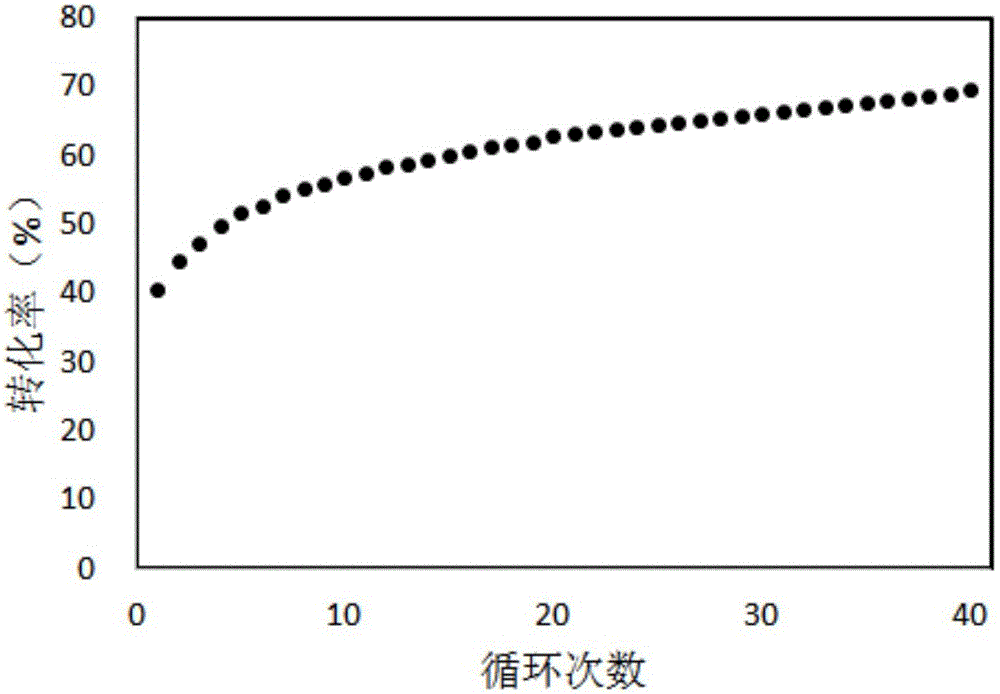
Method used
Image
Examples
preparation example Construction
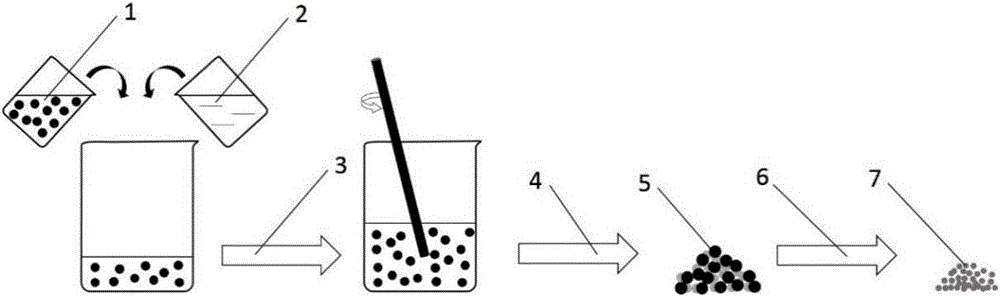
[0032] A lithium silicate CO 2 The preparation method of adsorbent comprises the following steps:
[0033] (1) Dissolving the organolithium salt and the silica sol, stirring at normal temperature or while heating over fire until the solvent is evaporated to dryness; the method of heating over fire can be a water bath or an oil bath.
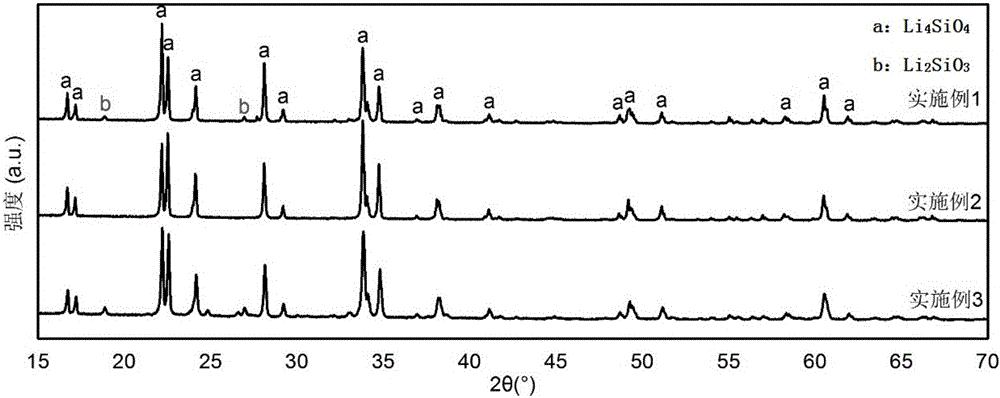
[0034] (2) In air or other oxygen-containing atmospheres, the evaporated solids are calcined in a muffle furnace at 550°C to 1200°C to obtain the desired lithium silicate CO 2 Adsorbent; in this process, organolithium salts are oxidized to lithium carbonate, which reacts with silica to form lithium silicate.
[0035] Wherein, the stoichiometric ratio of lithium in the organolithium salt to silicon dioxide in the silica sol is 4.0˜10:1. The content of silicon dioxide in the sol can be calculated by the mass fraction of silicon dioxide; sometimes the purity of organic lithium salt reagents is uncertain, and the content of lithium can be calculate...
Embodiment 1
[0038] (1) Taking the preparation of 5g lithium silicate as standard, 2LiCH 3 COO+4O 2 → Li 2 CO 3 +3CO 2 ↑+3H 2 O↑, and 2Li 2 CO 3 +SiO2 2 → Li 4 SiO 4 +2CO 2 The reaction formula of ↑ calculates that the mass of required silicon dioxide is 2.51g, and the mass of required lithium acetate is 11.01g;
[0039](2) The lithium acetate reagent is calculated according to the weight loss curve by a thermogravimetric analyzer to obtain its burning vector, and the content of lithium acetate in the lithium acetate reagent is further calculated to be 66.1%. Since lithium acetate needs to react with a coefficient of 1.05 times, and then calculate The quality of lithium acetate reagent required for the reaction is 11.01g×1.05÷66.1%=17.49g; and by the mass fraction of silica sol, the mass of required silica sol is calculated to be 8.35g;
[0040] (3) Dissolve the required lithium acetate reagent and neutral silica sol (containing 30% silicon dioxide) in 120mL deionized water (the...
Embodiment 2
[0045] Repeat Example 1 with the same steps as described, the difference is that in step 3, 9.1765g of lithium formate monohydrate reagent is used to replace the lithium acetate reagent, and the ratio of the stoichiometric number of lithium to silicon dioxide is 4.1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com