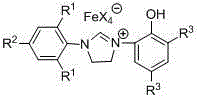
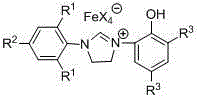
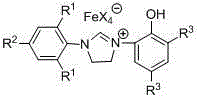
Ionic type iron (III) complex with monophenol functionalization tetrahydroglyoxaline cations and preparation method and application thereof
A technology for imidazoline cationic and ionic, which is applied in the field of ionic iron complexes and their preparation, and achieves the effects of simple reaction system, favorable industrial application, and easy product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment one: [(Ar 1 NCH 2 CH 2 NAr 2 )CH][FeX 4 ] (Ar 1 = 2,6-di-CH(CH 3 ) 2 -C 6 h 3 , Ar 2 = 2-(OH)-C 6 h 4 , X = Cl) for the synthesis of
[0038] Mix and dissolve 2,6-diisopropylaniline (10.0 ml, 48 mmol) and triethylamine (7.3 ml, 48 mmol) in dry tetrahydrofuran, slowly add oxalyl chloride monoethyl The ester (5.1 ml, 48 mmol) was stirred at room temperature for 5 hours after the addition was complete. After filtering, the filtrate was washed three times with dilute hydrochloric acid and saturated brine respectively, and the organic phase was dried with anhydrous sodium sulfate for 12 hours. The organic phase was concentrated to saturation, and 100 ml of n-hexane was added, a solid was precipitated, filtered, and dried to obtain a white solid ( N -(diisopropylphenyl)ethyl oxalate), the yield was 92%.
[0039] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room...
Embodiment 2
[0055] Embodiment two: [(Ar 1 NCH 2 CH 2 NAr 2 )CH][FeX 4 ] (Ar 1 = 2,6-di-CH(CH 3 ) 2 -C 6 h 3 , Ar 2 = 2-(OH)-C 6 h 4 , X = Br) synthesis of
[0056] In turn [(Ar 1 NCH 2 CH 2 NAr 2 )CH]Cl (0.36 g, 1.0 mmol) and NaBr (0.62 g, 6.0 mmol) were added to a tetrahydrofuran solution of iron tribromide (0.30 g, 1.0 mmol), reacted at 45°C for 16 hours, and vacuum pumped The solvent was removed, washed with hexane, drained, extracted with tetrahydrofuran, and the supernatant liquid was transferred by centrifugation. Hexane was added to the supernatant liquid for recrystallization, and reddish-brown crystals were precipitated at room temperature, with a yield of 93%.
[0057] Carry out elemental analysis to product, the result is as follows:
[0058] Elemental analysis
[0059] C:(%) H:(%) N:(%) theoretical value 36.09 3.89 4.01 actual value 36.32 4.15 3.92
[0060] This complex [(Ar 1 NCH 2 CH 2 NAr 2 )CH][FeBr 4 ] exists in ...
Embodiment 3
[0062] Embodiment three: [(Ar 1 NCH 2 CH 2 NAr 2 )CH][FeX 4 ] (Ar 1 = 2,6-di-CH(CH 3 ) 2 -C 6 h 3 , Ar 2 = 3,5-di-C(CH 3 ) 3 -2-(OH)-C 6 h 2 , X = Cl) for the synthesis of
[0063] [(Ar 1 NCH 2 CH 2 NAr 2 )CH]Cl was synthesized referring to the steps of Example 1, using 3,5-di-tert-butyl-2-hydroxyaniline instead of 2-aminophenol.
[0064] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl3, TMS): 8.27 (s, 1H), 7.47 (t, 1H), 7.36 (d, 1H), 7.31 (s, 1H), 7.29 (s, 1H), 6.96 (d, 1H), 4.92 (t, 2H), 4.48 (t, 2H), 3.51-3.40 (m, 2H), 1.44 (s, 9H), 1.37 (d, 6H), 1.30 (d, 15H)ppm.
[0065] Carry out elemental analysis to product, the result is as follows:
[0066] Elemental analysis
[0067] C:(%) H:(%) N:(%) theoretical value 73.93 9.20 5.95 actual value 73.72 8.96 5.88
[0068] Compound cationic part [(Ar ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com