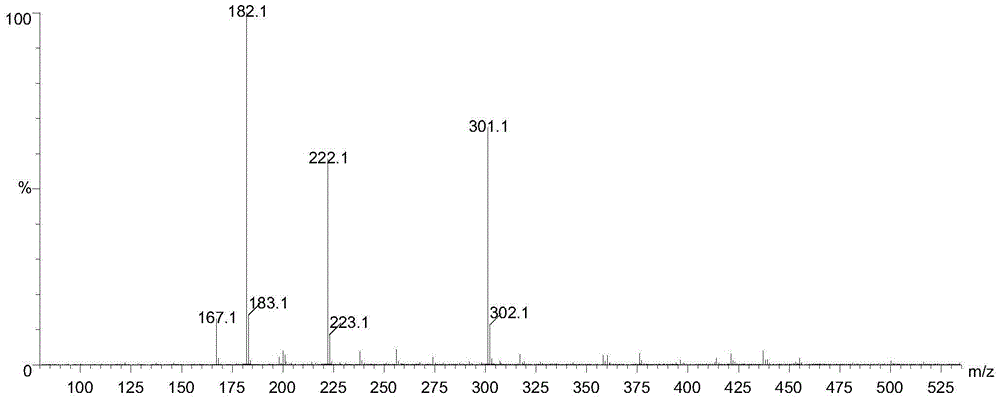
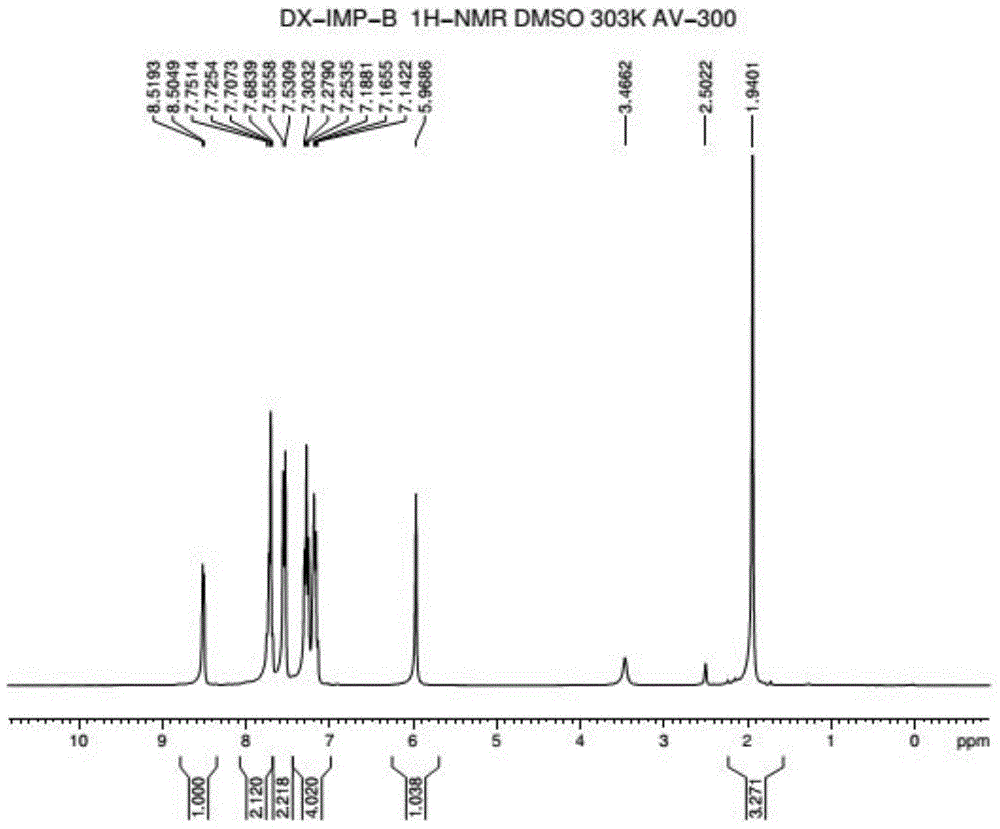
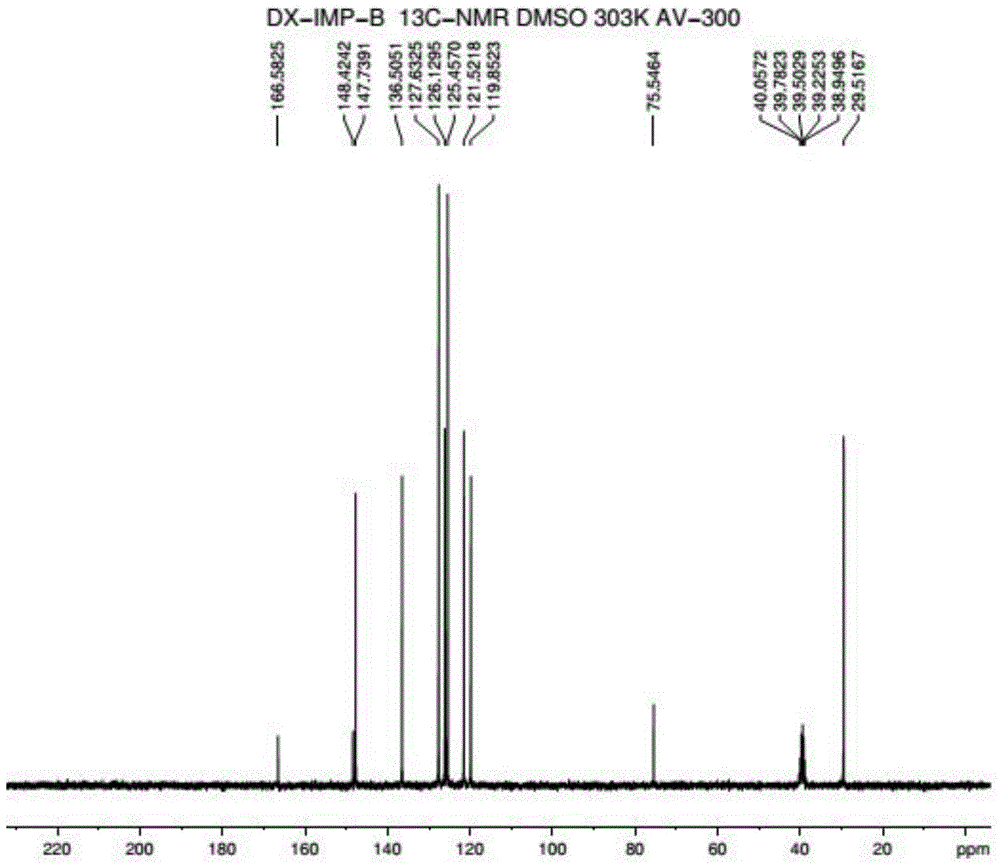
Method for preparing 2-pyridinemethanol-alpha-methyl-alpha-phenyl serving as intermediate of doxylamine succinate
A technology of pyridyl phenyl methyl carbinol and doxylamine succinate, applied in the field of medicine, can solve the problem that 2-pyridyl phenyl methyl carbinol cannot be industrialized, and achieves rapid reaction, high conversion rate, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The reaction equation is as follows:
[0032]
[0033] S1: Synthesis of 2-pyridylphenylmethylcarbinol
[0034] Add 10.5g of 2-benzoylpyridine into a 250ml three-necked round-bottomed flask under nitrogen protection, dissolve in 30ml of anhydrous tetrahydrofuran, place in a -25°C cold bath, cool down to -20°C, and slowly add dropwise to 1mol / L methylmagnesium bromide anhydrous tetrahydrofuran solution 85mL, control the internal temperature not greater than -15°C during the dropwise addition, keep warm and stir for 1.5~2h after the dropwise addition.
[0035] TLC determines the end point of the reaction, and the developing agent is: V 石油醚 :V 乙酸乙酯 =5:1.
[0036] After the reaction was completed, 200 ml of 10% ammonium chloride ice water solution was slowly added dropwise to the flask under the protection of nitrogen, and the addition was completed in 0.5 hours, and the stirring was continued for 0.5 hours. Add ethyl acetate, stand still to separate the liquids, kee...
Embodiment 2
[0041] The reaction equation is as follows:
[0042]
[0043] S1: Synthesis of 2-pyridylphenylmethylcarbinol
[0044] Under nitrogen protection, add 10.5g of 2-benzoylpyridine into a 250ml three-neck round-bottomed flask, dissolve in 30ml of anhydrous tetrahydrofuran, put it in a -20°C cold bath, cool down to -20~-15°C, and slowly drop Add to 85 mL of 1 mol / L methylmagnesium chloride anhydrous tetrahydrofuran solution, control the internal temperature not to exceed -10°C during the dropwise addition, and keep stirring for 1.5 to 2 hours after the dropwise addition.
[0045] TLC determines the end point of the reaction, and the developing agent is: V 石油醚 :V 乙酸乙酯 =5:1.
[0046] After the reaction was completed, 200 ml of ammonium chloride ice-water solution was slowly added dropwise to the flask under the protection of nitrogen, and the addition was completed in 0.5 hours, and the stirring was continued for 0.5 hours. Add ethyl acetate, let stand to separate the liquids, ...
Embodiment 3
[0051] The reaction equation is as follows:
[0052]
[0053] S1: Synthesis of 2-pyridylphenylmethylcarbinol
[0054] Add 10.5g of 2-benzoylpyridine into a 250ml three-neck round-bottomed flask under nitrogen protection, dissolve in 30ml of anhydrous methyl tetrahydrofuran, place in a -25°C cold bath, cool down until the internal temperature reaches -20°C, and slowly add Add to 85mL of 3mol / L methylmagnesium bromide 2-methyltetrahydrofuran solution, control the internal temperature not to exceed -15°C during the dropwise addition, and keep stirring for 1.5-2h after the dropwise addition.
[0055] The end point of the reaction was determined by TLC, and the developer was: V petroleum ether: V ethyl acetate = 5:1.
[0056] After the reaction was completed, 200 ml of ammonium chloride ice-water solution was slowly added dropwise to the flask under the protection of nitrogen, and the addition was completed in 0.5 hours, and the stirring was continued for 0.5 hours. Add ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com