Preparing method for Ba/Fe/Na metal and carboxylic acid Schiff base complex
A technology of complexes and Schiff bases, applied in the field of chemistry, can solve the problems of unreported triheterometallic complexes, and achieve the effects of simple preparation methods, strong repeatability, and stable product performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take 0.12 mmol barium chloride dihydrate, 0.03 mmol ferric chloride hexahydrate, 0.06 mmol sodium chloride and H 4 0.03 mmol of L ligand, put in a 15 mL polytetrafluoroethylene reactor, use 1 mL of dimethylformamide, 4 mL of ethanol and 2 mL of water as a mixed solvent, place in an oven, and heat to 100 ℃ for 72 hours , And then slowly drop to room temperature to obtain brown crystals with a yield of 31%.
Embodiment 2
[0022] Take 0.12 mmol barium chloride dihydrate, 0.03 mmol ferric chloride hexahydrate, 0.06 mmol sodium chloride and H 4 L ligand 0.03 mmol, put it in a 15 mL polytetrafluoroethylene reactor, use 3 mL dimethylformamide, 2 mL ethanol and 2 mL water as a mixed solvent, place it in an oven, and heat to 110 ℃ for 72 hours , And then slowly drop to room temperature to obtain brown crystals with a yield of 10%.
[0023] The main infrared absorption peaks are: 3394 (m), 2932 (w), 2858 (w), 1607 (w), 1565 (w), 1388 (w), 1308 (w), 1286 (w), 1217 (w) ), 1122 (m), 1027 (s), 978 (s), 952 (s), 919 (s), 861 (s), 794 (m), 760 (m), 712 (m), 582 (m ), 558 (m), 525 (m), 502 (s), 466 (m), 421 (s).
[0024] Related characterization of the complex
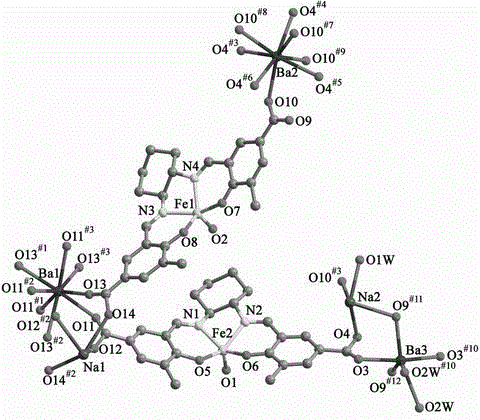
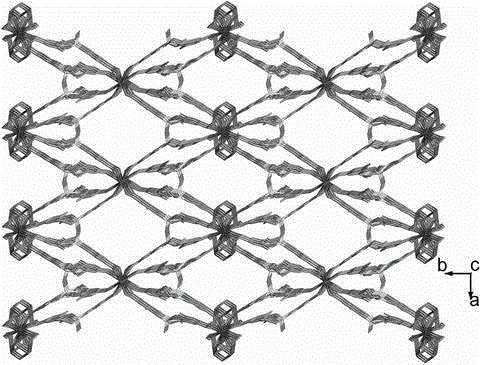
[0025] (1) Determination of the crystal structure of the complex
[0026] The diffraction data of the complex was collected on the Oxford Diffraction Gemini R Ultra diffractometer, 293 K, Mo K α Ray (λ = 0.71069 ?). Use technical scans for correction. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com