Narrowly-dispersed phenol-phenolic varnish resin preparation method and narrowly-dispersed phenol-phenolic varnish resin obtained by means of the preparation method
A technology of phenol novolac and manufacturing method, which is applied in the direction of coating, etc., can solve the problems of deterioration of productivity, increase of process, and inability to obtain phenol novolac resin, etc., and achieve the beneficial effects of small environmental burden and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (preparation process)
[0068] In a stirred tank type reactor equipped with a stirrer, a temperature regulator, a reflux condenser, a total condenser, a decompression device, etc., add 100 parts of bisphenol F and 600 parts of phenol were heated up to 80° C., then 1.55 parts of oxalic acid dihydrate was added, stirred and dissolved for 10 minutes, and 115 parts of 37.5% formalin were added dropwise over 30 minutes. Thereafter, the reaction was continuously performed for 3 hours while maintaining the reaction temperature at 92°C. After the reaction was terminated, the temperature was increased to 110°C for dehydration, and about 90% of the residual phenol was recovered under the recovery conditions of 150°C and 60mmHg, then recovered under the recovery conditions of 160°C and 5mmHg, and then further recovered at 160°C 10 parts of water were dripped over 90 minutes under conditions of C and 80 mmHg to remove residual phenol, and a crude phenol novolac resin was obtained....
Embodiment 2
[0073] (preparation process)
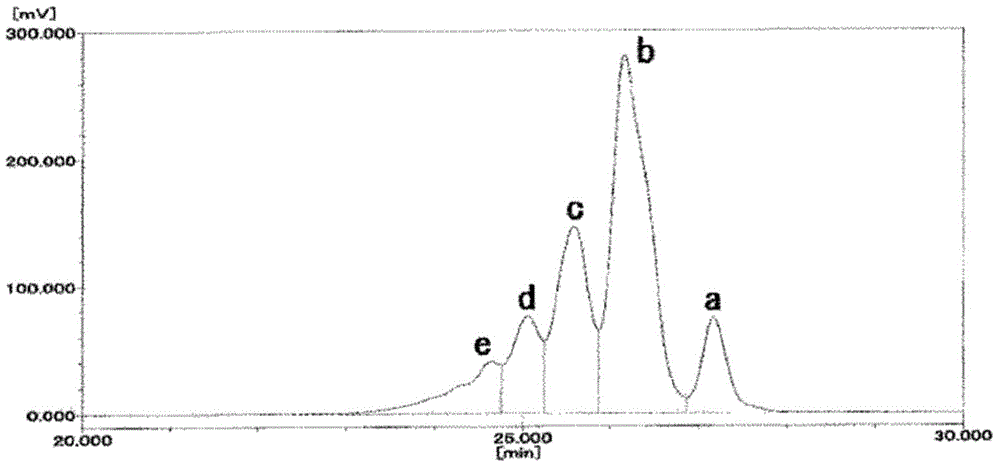
[0074] 210 parts of evaporated components obtained in Example 1 were used as bisphenol F, 680 parts of phenol, 1.78 parts of oxalic acid dihydrate, and 115 parts of 37.5% formalin. Exactly the same operation as in Example 1 was performed to obtain a crude phenol novolac resin. Regarding the composition of the obtained crude phenol novolak resin, the amount of 2-nuclear bodies is 52.8 area%, the amount of 3-nuclei bodies is 25.0 area%, the amount of 4-nuclei bodies is 12.7 area%, the amount of 5-nuclei bodies is 5.2 area%, and the amount of 6-nuclei bodies is 5.2 area%. The content above the bulk was 4.3 area%, Mw was 347, and the degree of dispersion (Mw / Mn) was 1.192.
[0075] (distillation process)
[0076] The obtained crude phenol novolac resin was continuously supplied at 10 kg / h to the centrifugal thin film evaporator used in Example 1 for 1 hour, and the evaporated components and phenol novolac resin were continuously extracted. Regardi...
Embodiment 3
[0079] (preparation process)
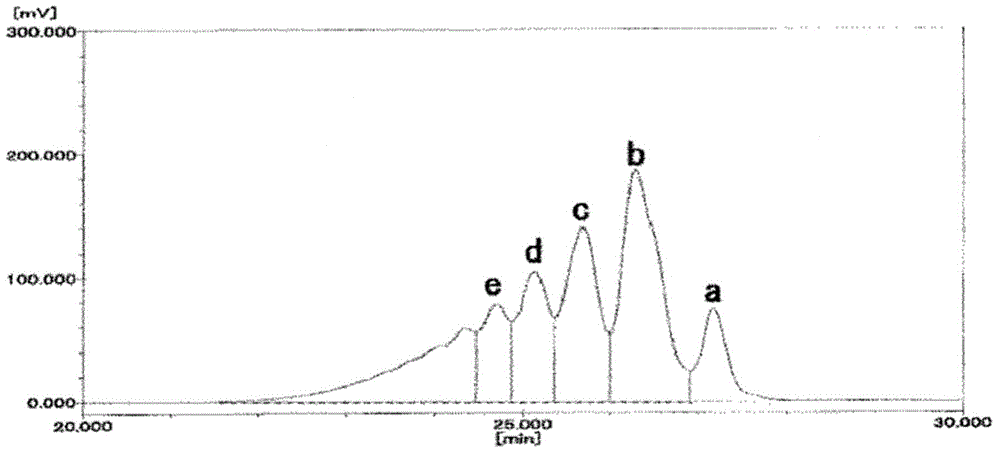
[0080] 25 parts of evaporated components obtained in Example 2 were used as bisphenol F, phenol was changed to 600 parts, oxalic acid dihydrate was changed to 1.25 parts, and 37.5% formalin was changed to 170 parts. Exactly the same operation as in Example 1 was performed to obtain a crude phenol novolac resin. Regarding the composition of the obtained crude phenol novolak resin, the amount of 2-nuclei was 38.0 area%, the amount of 3-nuclei was 24.5 area%, the amount of 4-nuclei was 20.4 area%, the amount of 5-nuclei was 9.3 area%, and the amount of 6-nuclei was 9.3% by area. The content above the bulk was 7.8 area%, Mw was 403, and the degree of dispersion (Mw / Mn) was 1.195.
[0081] (distillation process)
[0082] To the centrifugal thin-film evaporator used in Example 1, the obtained crude phenol novolak resin was continuously supplied at 31 kg / h, and the evaporated components and phenol novolak resin were continuously extracted. Regarding ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com