shRNA transgenic recombinant plasmid for inhibiting swine influenza viruses and use thereof
A technology of swine influenza virus and recombinant plasmid, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Construction of shRNA transgene recombinant plasmid capable of inhibiting swine influenza virus replication and infection
[0041] 1. Design of shRNA target sites and primers that can inhibit the replication and infection of swine influenza virus
[0042] In this example, the shRNA that can inhibit the replication and infection of swine influenza virus is based on the genome sequence of influenza virus, and selects PA, PB1, PB2 and NP, which are highly conserved in influenza virus, as target sequences to design and synthesize siRNAs, as shown in Table 1. . The specific positions of the selected conserved sequences (indicated by numbers in the primers) are the corresponding siRNAs. The selected target gene fragments are named after the original gene.
[0043] Table 1 shRNA target sites and primer design
[0044]
[0045] 2. Construction of NP, PB2, PB1 and PA gene shRNA expression cassettes
[0046] Dissolve single-stranded DNA primers (2OD) in 60 μL ann...
Embodiment 2
[0050] Example 2 Construction of recombinant lentiviral vector and establishment of cell line stably expressing shRNA
[0051] 1. Construction of recombinant lentiviral vector
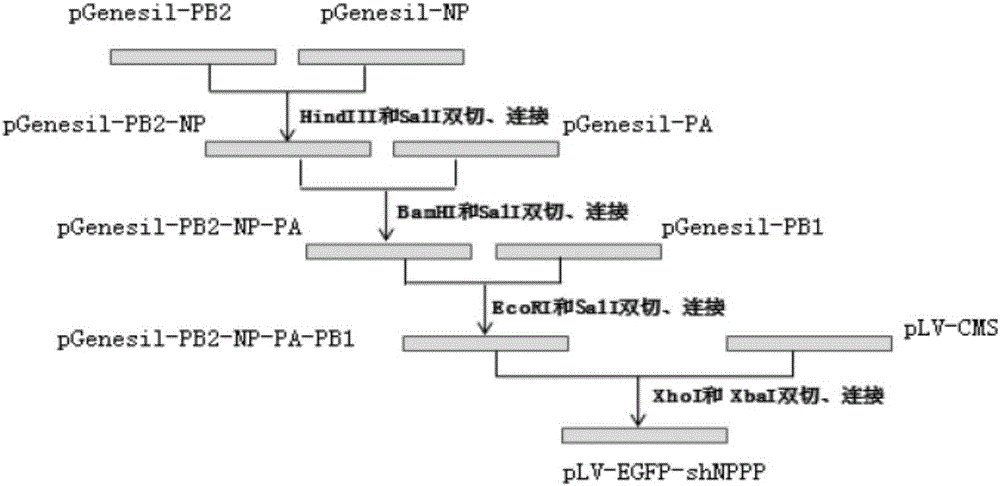
[0052] The pLV-EGFP lentiviral vector was double-digested with ClaI and XbaI, and the large fragment recovered from the gel was connected to the synthetic multiple cloning site 5'-ClaI-Xmal-XhoI-MluI-XbaI-3', and the constructed lentiviral expression vector was named pLV-MCS, the expression cassette vector and lentiviral expression vector were cut with XhoI and XbaI, named pLV-EGFP-shNPAPB1PB2, the three-gene expression cassette and lentiviral expression vector were named pLV-EGFP-shPAPB1PB2, pLV-EGFP-shNPAPB1 , pLV-EGFP-shNPAPB2, pLV-EGFP-shNPB1PB2, the construction principle is as follows figure 1 shown.
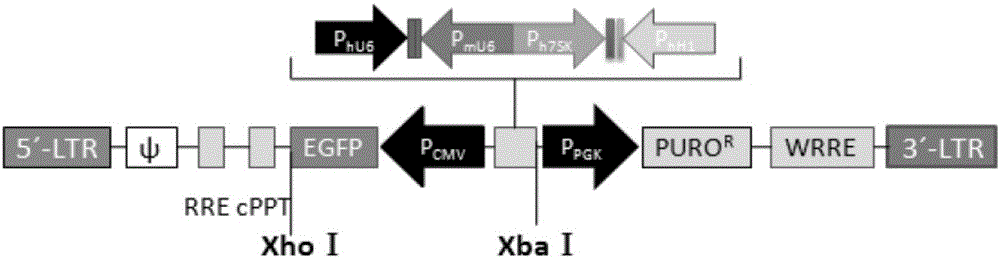
[0053] The tandem shRNA gene and reporter gene arrangement constructed in the present invention are as follows figure 2 shown. Three-gene shRNA and four-gene shRNA are arranged in tandem on th...
Embodiment 3
[0059] Example 3 Comparison of three-gene and four-gene cell lines inhibiting the replication of H1N1 swine influenza virus
[0060] 6 passages of shPAPB1PB2-MDCK, shNPAPB1-MDCK, shNPAPB2-MDCK, shNPB1PB2-MDCK, shNPAPB1PB2-MDCK, Mock-MDCK and MDCK that were subcultured synchronously, with 1×10 5 The number of cells was planted in a 12-well cell culture plate. When the cell confluence reached 70%-80%, it was diluted with infection medium (penicillin 100 U / mL, streptomycin 100 μg / mL, TPCK-Trypsin 4 μg / mL) H1N1 swine influenza virus (LM / 2004 strain, 1000TCID 50 ) to inoculate cells, incubate at room temperature for 1 hour, remove virus dilution, add 1 mL infection medium to each well, and absorb cell supernatant at 12, 24, 36, 48, and 60 hours after infection to make 10 -1 -10 -8 Gradual dilution, each dilution was inoculated with three 9-10-day-old common chicken embryos, and after 72 hours (LM / 2004), the allantoic fluid was drawn to detect the virus titer.
[0061] H1N1 swine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com