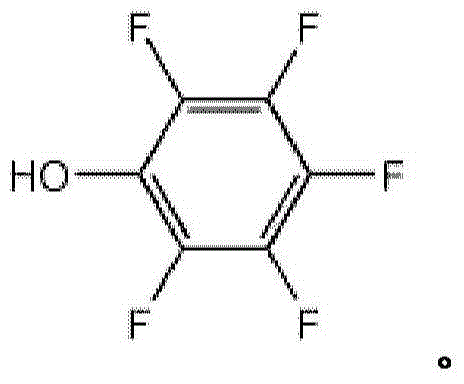
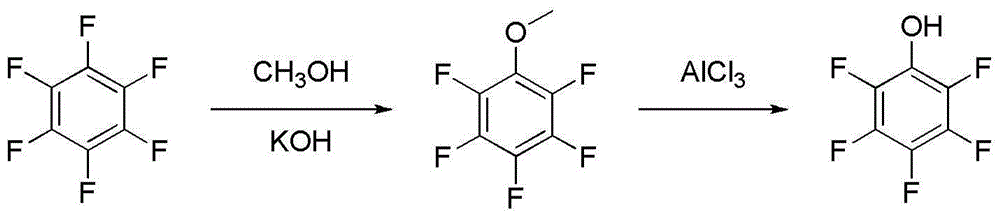
Preparation method for pentafluorophenol
A technology of pentafluorophenol and pentafluoroanisole, which is applied in the preparation of organic compounds, ether preparation, chemical instruments and methods, etc. It can solve the problem of easy oxidation and hydrolysis of sodium methoxide, inability to scale up industrial production, difficulty in controlling reaction temperature, etc. problems, to achieve the effect of easy control of reaction temperature, avoiding high temperature and pressurized conditions, and solving stirring problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the preparation technology of pentafluorophenol
[0094] Step 1: Preparation of pentafluoroanisole (etherification)
[0095] Add hexafluorobenzene (146g) into a 1L four-neck flask, add potassium hydroxide methanol solution (potassium hydroxide 46g, methanol 138g) dropwise at 35-45°C, and continue the reaction for 5-10 hours after the addition is complete. After the reaction, cool down to room temperature, add water, separate layers, rectify the organic layer, and collect fractions at 138-139°C to obtain pentafluoroanisole (127g), which is a colorless transparent liquid. Yield 82%.
[0096] Step 2: Preparation (cracking) of pentafluorophenol
[0097] Add 1,2-dichloroethane (190g) and aluminum trichloride (95g) into a 1L four-neck flask, add pentafluoroanisole (117g) dropwise at 50-60°C, and continue the reaction for 4 hours after the addition is completed. After the reaction, cool down to room temperature, add water, separate layers, rectify the organic ...
Embodiment 2
[0100] Embodiment 2: the preparation technology of pentafluorophenol (hexafluorobenzene one-step method prepares pentafluorophenol)
[0101] Add tert-butanol (500g), potassium hydroxide (75g) and hexafluorobenzene (100g) into a 2L four-neck flask, and react at 60-70°C for 3 hours. After the reaction, cool down to room temperature, add water, distill off the solvent, acidify the aqueous layer with hydrochloric acid, extract with methyl tert-butyl ether, combine the organic layers and rectify, distill off the solvent first, and then collect fractions at 142-144°C. Pentafluorophenol (69 g) was obtained as a white to light brown solid with a melting point of 34-36° C. and a yield of 70%.
[0102] The synthetic route of pentafluorophenol in this preparation technology is as follows:
[0103]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com