Preparation method for vortioxetine
A compound and reagent technology, applied in the field of vortioxetine phenyl) piperazine, can solve the problems of unsuitable industrial operation, high cost of industrialization, difficult procurement, etc., achieve significant creativity and practical application value, convenient procurement, and reduce costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
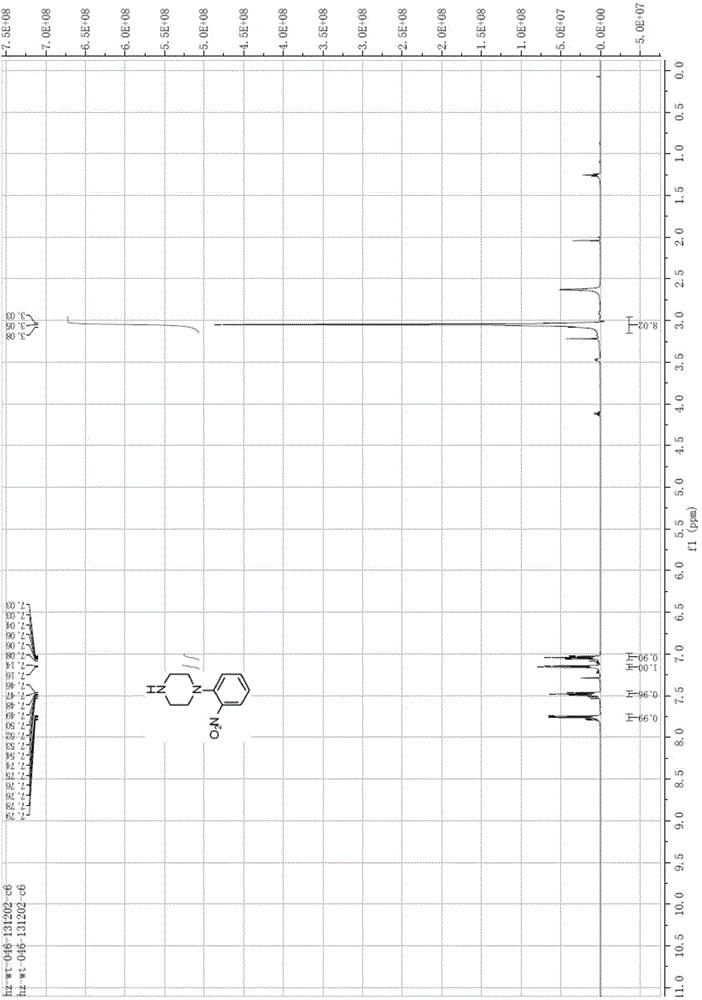
[0084] Preparation of 1-(2-nitrophenyl)piperazine (Compound 2)
[0085] Dissolve o-fluoronitrobenzene (compound 1) (151.00g, 1.07mol, 1.0eq) and piperazine (368.74g, 4.28mol, 4.0eq) in ethanol (800ml), stir at room temperature for 2 hours, concentrate, add 1000ml of water , extracted with ethyl acetate, the organic phases were combined, washed with water, washed with salt, and concentrated to obtain a total of 219 g of red liquid with a molar yield of 98.75%.
[0086] EI-MS[M+1]=208.0;
[0087] 1 HNMR (CDCl3), δHppm: 2.63 (s, 1H), 3.05 (s, 8H), 7.03-7.06 (td, 1H), 7.14-7.16 (dd, 1H), 7.46-7.50 (td, 1H), 7.74- 7.76(dd,1H), see figure 1 .
Embodiment 2
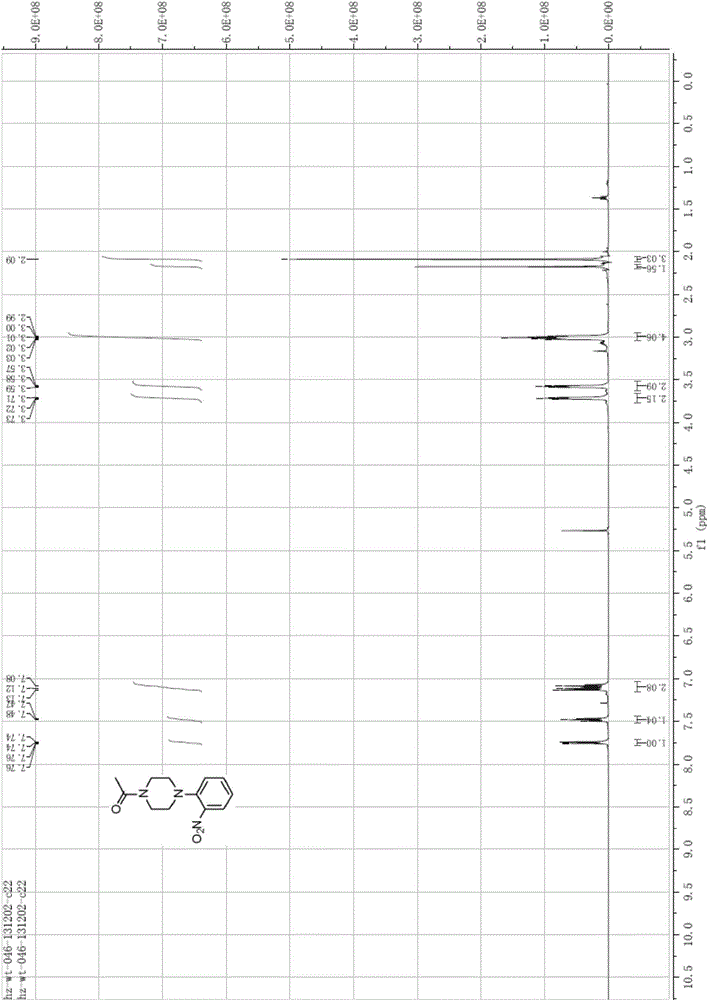
[0089] Preparation of 1-acetyl-4-(2-nitrophenyl)piperazine (Compound 3)
[0090] Compound 2 (153.80g, 0.74mol, 1.0eq) was dissolved in dichloromethane (1000ml), triethylamine (127.43g, 1.26mol, 1.7eq) was added, cooled to about 0°C, and acetic anhydride (113.65g , 1.11mol, 1.5eq), the temperature was controlled below 20 degrees, stirred for 30min, the organic phase was washed with saturated sodium bicarbonate, water, saturated brine, and concentrated to obtain a total of 179g of red solids with a yield of 96.8%, without purification, directly Go to the next step.
[0091] EI-MS [M+1] = 250.3;
[0092] 1 HNMR (CDCl3), δHppm: 2.09(s, 3H), 2.99-3.03(m, 4H), 3.57-3.59(t, 2H), 3.71-3.73(t, 2H), 7.08-7.13(m, 2H), 7.46-7.50(td,1H), 7.74-7.76(dd,1H), see figure 2 .
Embodiment 3
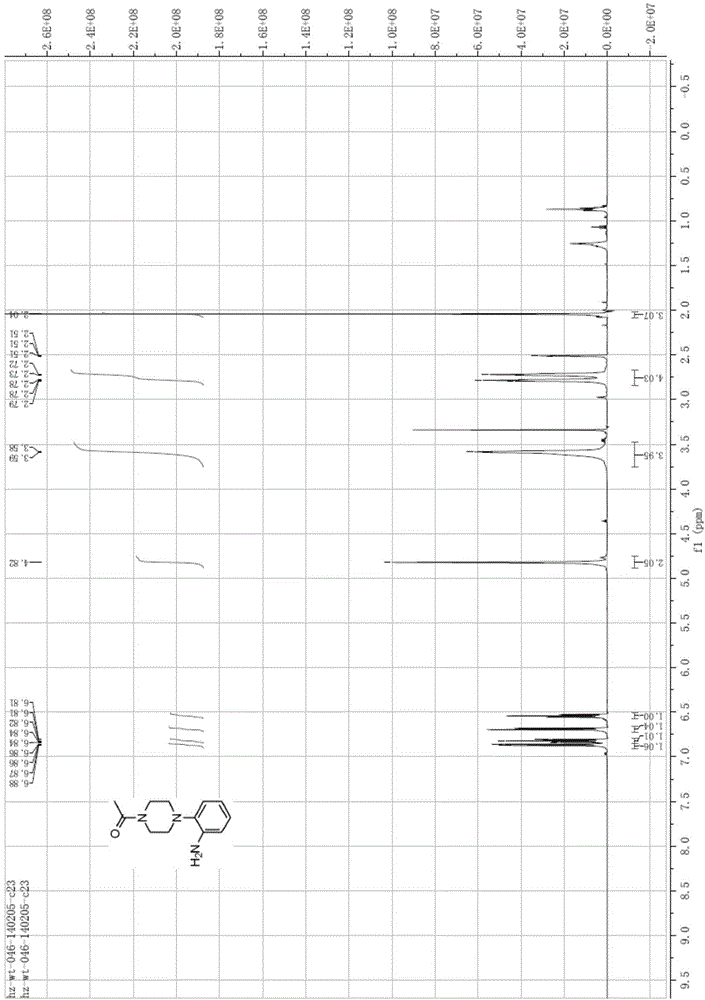
[0094] Preparation of 1-acetyl-4-(2-aminophenyl)piperazine (compound 4)
[0095] Compound 3 (217.57g, 0.87mol, 1.0eq) was dissolved in ethanol (1200ml), hydrogenated at room temperature under 5kg pressure, reacted overnight, concentrated, and the concentrated solid was recrystallized with ethanol and n-hexane to obtain a total of 175.79g of pure product, The total yield is 91.85%.
[0096] EI-MS[M+1]=220.3;
[0097] 1 HNMR (CDCl3), δHppm: 2.13(s, 3H), 2.90-2.93(m, 4H), 3.62-3.63(m, 2H), 3.71-3.73(t, 2H), 6.82-6.83(td, 1H), 6.88-6.90(dd,1H), 6.99-7.00(m,2H), see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com