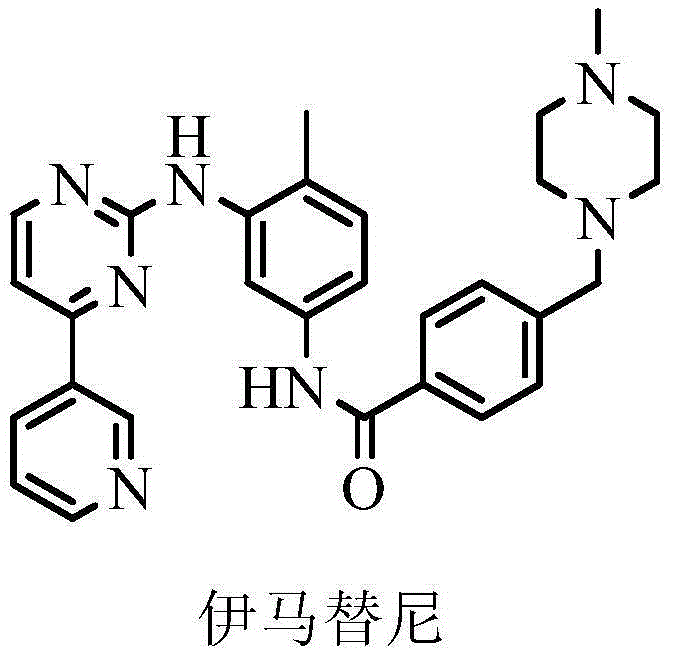
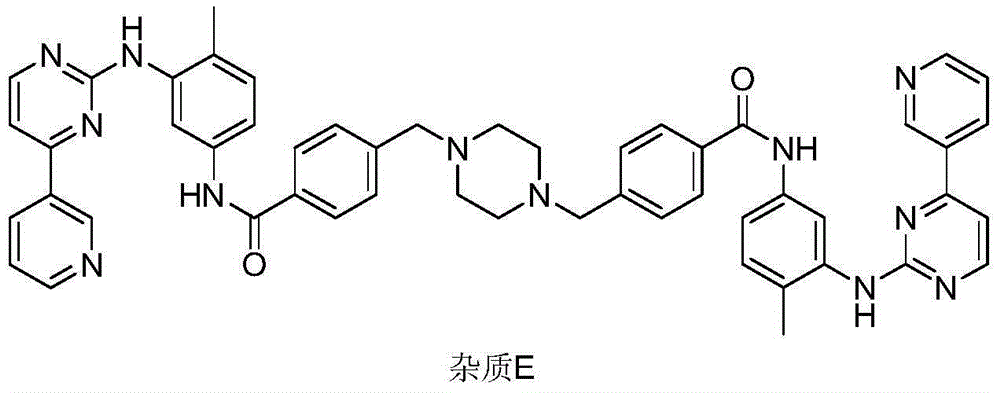
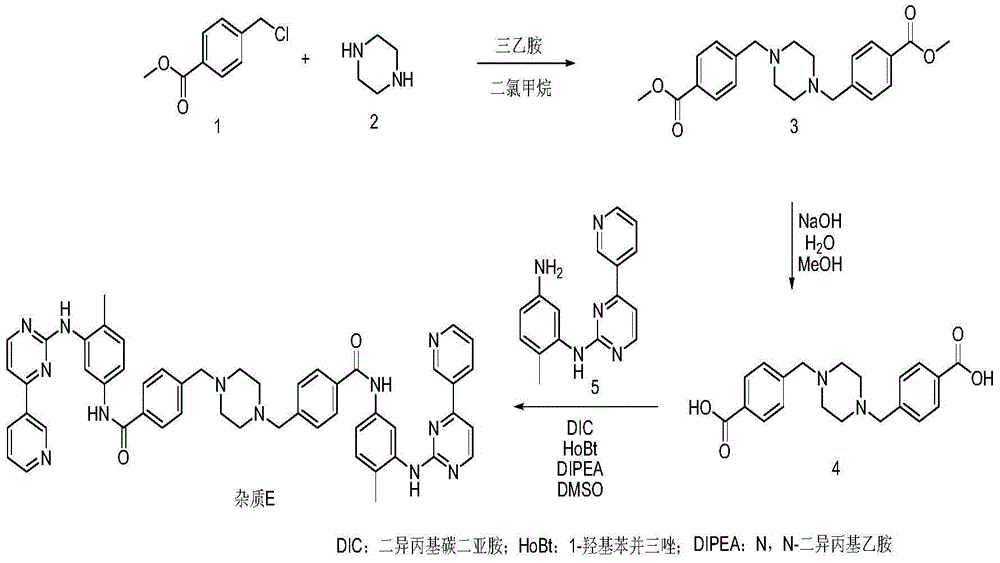
Preparation method of imatinib impurity
A technology of imatinib and impurities, which is applied in the field of preparation of impurities, can solve the problems of compound synthesis methods that have not been reported in literature, and achieve the effects of improving drug safety, simple preparation methods, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 26.0 g of methyl p-chloromethylbenzoate in 400 ml of dichloromethane, add 55 g of triethylamine with stirring at room temperature, slowly add 6.1 g of piperazine after the addition, and slowly raise the temperature to reflux for 4 hours after the addition. The temperature was lowered, the system was washed three times with 200ml of purified water, dried over anhydrous sodium sulfate and then spin-dried to obtain 24g of intermediate 3. Dissolve 25.2g of sodium hydroxide in 480ml of water, add 24g of intermediate 3, raise the temperature to 60°C and stir the reaction until the system is dissolved, adjust the pH=2~3 to precipitate a white solid, filter and dry to obtain 19.2g of compound 4, according to the purity of 100 % carry out the next step reaction.
Embodiment 2
[0022] Add 9.6g of compound 4 and 150ml of dimethyl sulfoxide into a round bottom flask, add 6.6g of 1-hydroxybenzotriazole, 7.0g of N,N-diisopropylcarbodiimide, add N, 10.5g of N-diisopropylethylamine, heated up to 60°C, stirred until completely dissolved, then added 15.8g of imaamine and stirred at constant temperature until the reaction did not change. After cooling down to room temperature, the reaction solution was poured into ice water, A yellow solid precipitated out and was filtered with suction to obtain a yellow solid. Disperse the obtained solid in water, add hydrochloric acid to adjust the pH to 3-5, filter with suction and dry. 18.2 g of impurity E was obtained by beating with methanol, with a molar yield of 76.8% and a purity of 96.4%.
Embodiment 3
[0024] Add 9.6g of compound 4 and 150ml of dimethyl sulfoxide into a round bottom flask, add 4.8g of 1-hydroxybenzotriazole, 5.3g of N,N-diisopropylcarbodiimide, add N, 8.6g of N-diisopropylethylamine, heated up to 60°C, stirred until completely dissolved, then added 17.8g of imamamine and stirred at constant temperature until no change, lowered to room temperature, poured the reaction solution into ice water, and precipitated A yellow solid was obtained by suction filtration to obtain a yellow solid. Disperse the obtained solid in water, add hydrochloric acid to adjust the pH to 3-5, filter with suction and dry. 17.2 g of impurity E was obtained by beating with methanol, with a molar yield of 72.6% and a purity of 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com