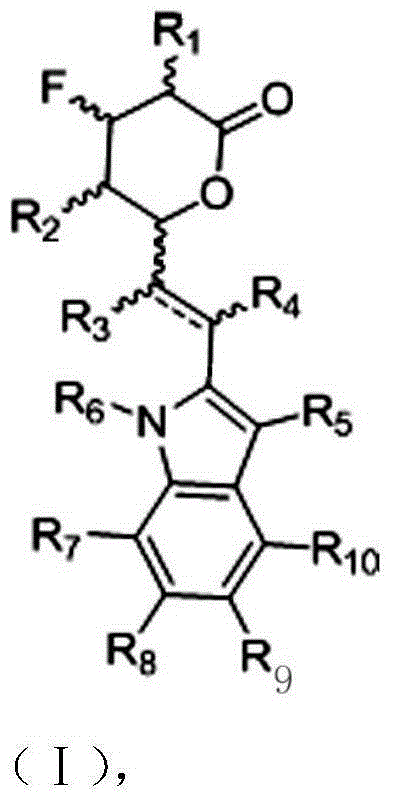
Polysubstituted indole statin fluorine-containing modifier and use thereof
An indole and substituent technology, applied in the field of medicinal chemistry, can solve the problems of rhabdomyolysis, carcinogenic toxicity, easy to cause liver disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Example 1 Preparation of fluvastatin lactone and reduction of fluvastatin lactone
[0129]Weigh 5.00g of fluvastatin sodium salt, add it to a 250ml eggplant-shaped bottle, add 100ml of dichloromethane and about 10ml of dilute hydrochloric acid diluted with 20 times concentrated hydrochloric acid, shake vigorously, and let stand to separate layers. Take the upper aqueous phase, and when the pH test paper detects and controls it at 2-3, it can be considered to be completely free. Liquid separation, 100ml dichloromethane equal volume extraction three times, combined lower organic phase, 20ml water backwash once, organic phase dried with anhydrous sodium sulfate. Vacuum spin dry at 45°C, evacuate with an oil pump until white bubbles appear, cool, and crush to obtain 4.23 g of white powder, the crude fluvastatin carboxylic acid is used in the next step without refining.
[0130] Weigh 4.00g of the above-mentioned fluvastatin carboxylic acid crude product, add it to a 250ml ...
Embodiment 2
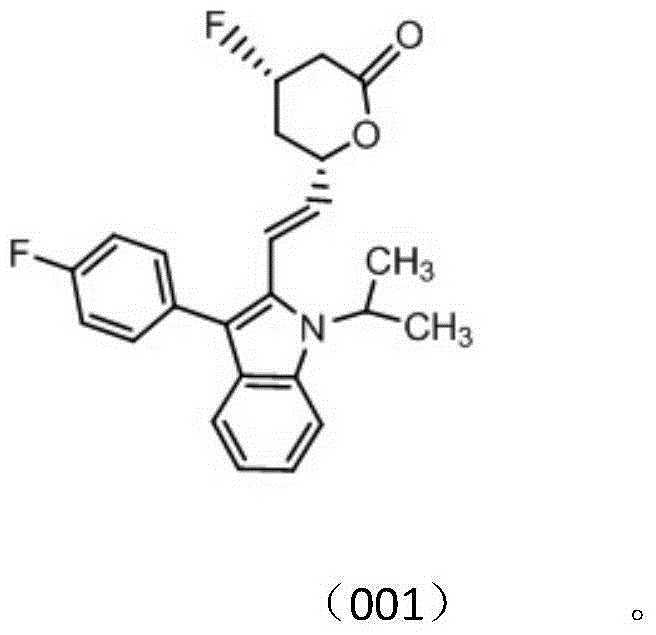
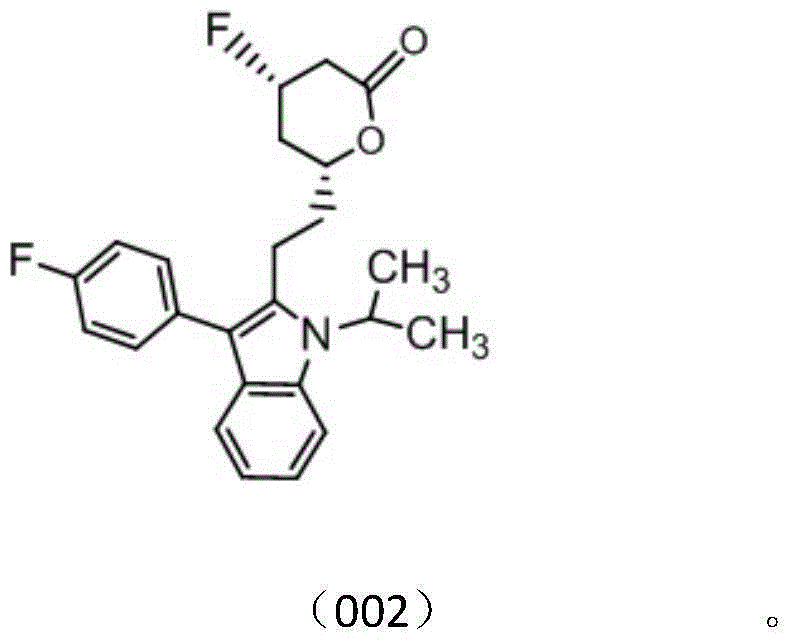
[0132] Example 2 Preparation of Compounds 001 and 002
[0133] Add a magnetic stirring bar of appropriate size into a 50ml reaction tube, replace the air and protect it with nitrogen, inject 30ml of dichloromethane, put the reaction vessel into a low-temperature stirring reaction bath (below -65°C), and inject 0.75ml of Diethylaminosulfur trifluoride, after stirring for about 15 minutes, a solution of 1.50 g of fluvastatin dissolved in 10 ml of dichloromethane was added slowly. After stirring and reacting for about 30 minutes, about 0.3 ml of triethylamine was added by injection, and after 2 hours, the temperature was naturally raised to react overnight. After the completion of the reaction was monitored by thin-layer chromatography, suction filtration, the filtrate was dried over anhydrous sodium sulfate, spin-dried, and separated by silver nitrate complexed silica gel column chromatography (gradient elution of isopropanol / petroleum ether) to obtain the fluvastatin fluorinate...
Embodiment 3
[0134] Example 3 Preparation of Compounds 003, 004, 005, 006
[0135] Take 1.00 g of fluvastatin fluoride (001), dissolve it in 6 ml of tetrahydrofuran, and put it in ice bath, add 1.5 ml of 1 mol / L LiOH solution and stir for 2 hours, acidify it with 10% hydrochloric acid until the pH is 2-3, and then depressurize Evaporate the solvent at 45°C, add about 10ml of acetone to dissolve, then slowly add 10% Na 2 CO 3 Aqueous solution, flocs and turbidity can be seen, add dropwise until flocs no longer appear. Heat until the turbidity dissolves, let it stand, and cool down slowly overnight. The next day, 0.68 g of needle crystals were obtained, namely fluvastatin fluoride sodium salt (003). The same method can be used to obtain the reduced fluoride sodium salt 004.
[0136] Get 1.00 g of fluvastatin fluoride (001), dissolve it in 6 ml of tetrahydrofuran, and put it in an ice bath, add 1.5 ml of 1 mol / L LiOH solution and stir for 2 hours, then acidify it with 10% hydrochloric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com