High-purity ptyltetracid dianhydride and synthesis method thereof, and polyimides synthesized on basis of ptyltetracid dianhydride
A technology of pterene tetra-acid dianhydride and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high loss of dianhydride compounds and the inability to recrystallize and purify dianhydride, so as to reduce the force, improve the glass transition temperature, and rigidity increased effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
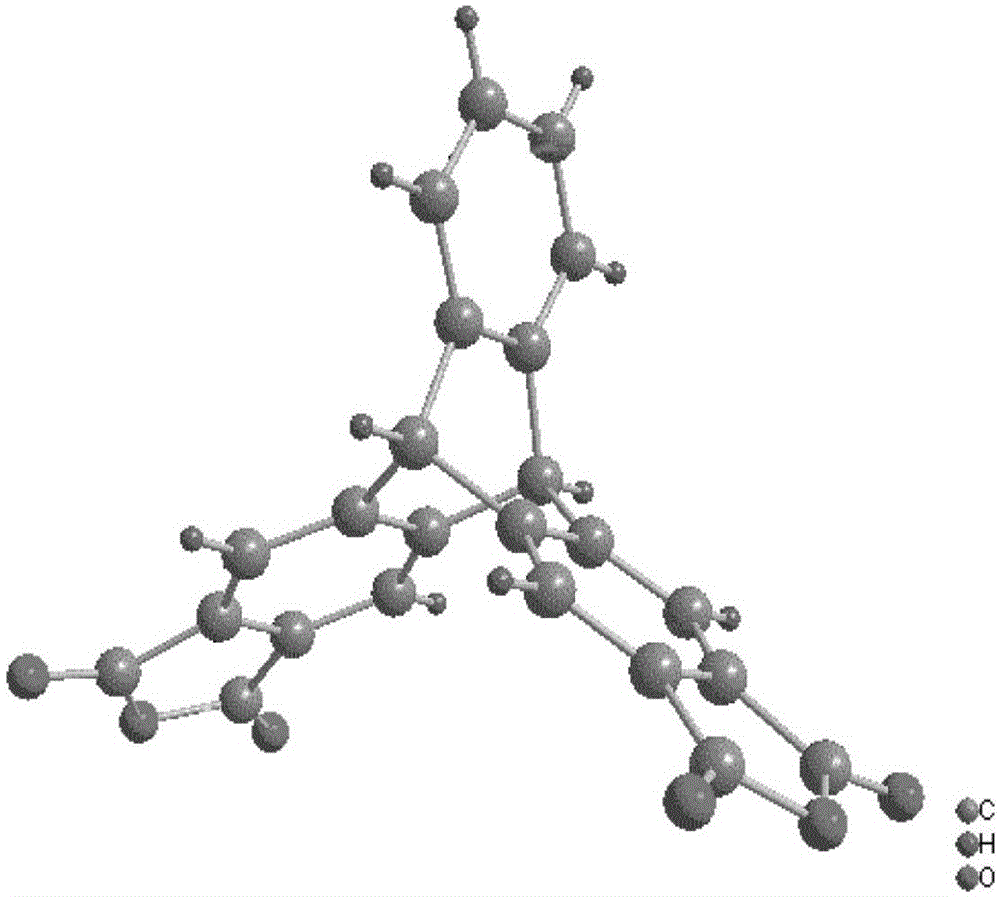
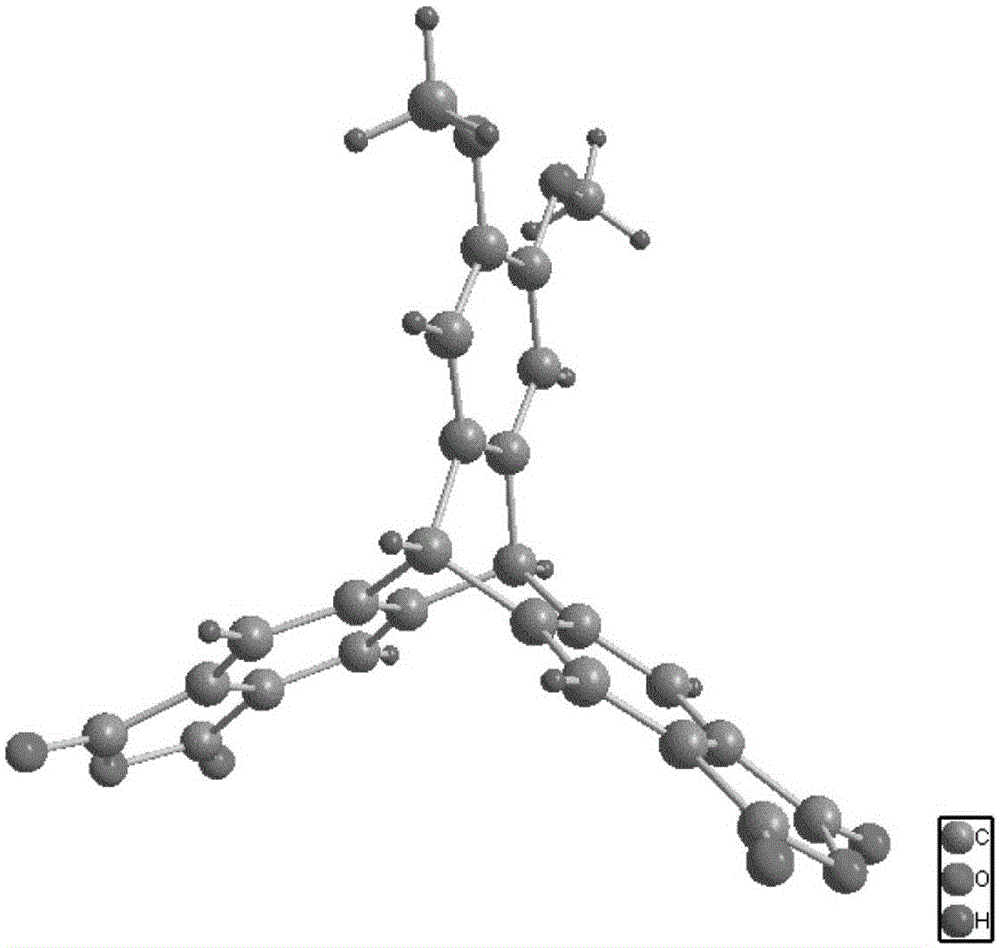
Image
Examples
Embodiment 1
[0075] Example 1: Preparation of high-purity 2,3,6,7-triptycene tetraacid dianhydride (TM1)
[0076] Under the conditions of ice-salt bath and vigorous stirring, in a 250ml three-necked flask with 120ml of o-xylene and 70ml of dichloromethane, add 60g of aluminum trichloride in four times, and keep the system temperature at 0°C during the addition. After the addition, react at room temperature for 0.5 h, and then react in a water bath at 65° C. for 4 h. Afterwards, the mixed reactant was slowly dispersed in 300ml of 5% hydrochloric acid ice-water mixed solution. During the pouring process, vigorous stirring was required. After standing still, suction filtration, the filter cake was washed with anhydrous acetone, dried, and o-xylene was recrystallized to obtain white 20g of flaky 2,3,6,7-tetramethylanthracene solid, with a melting point of 299°C.
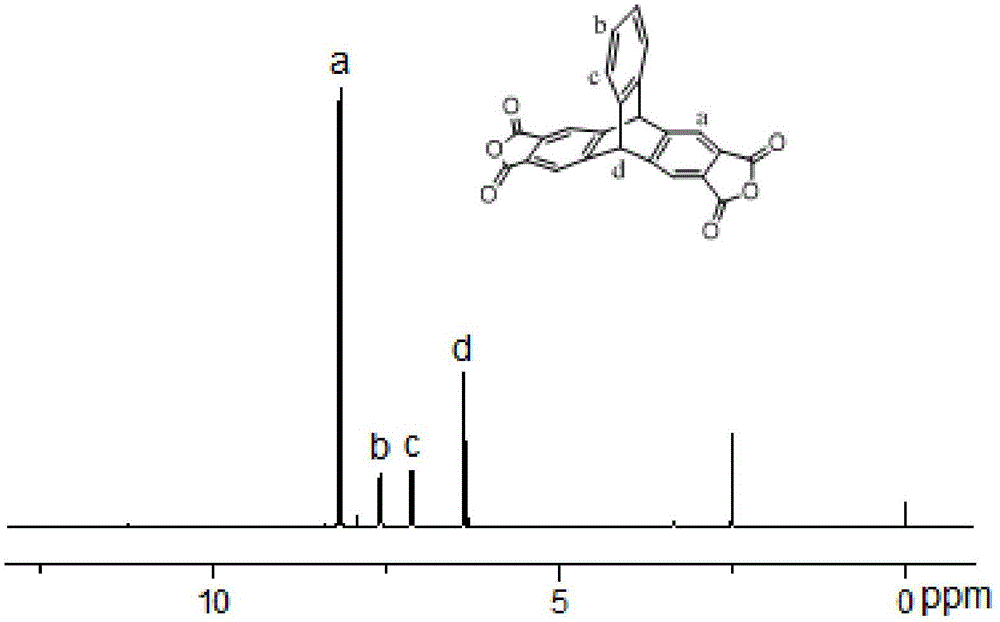
[0077] 1 HNMR (400MHz, CDCl3): δ=2.43(s,12H),7.68(s,4H),8.13(s,2H)
[0078] Weigh 2g of 2,3,6,7-tetramethylanthracene into a 500...
Embodiment 2
[0099] Example 2: Preparation of high-purity 13,14,15,16-tetrachloro-2,3,6,7-triptycene tetraacid dianhydride (TM2)
[0100] Under the conditions of ice-salt bath and vigorous stirring, in a 250ml three-neck flask with 120ml of o-xylene and 70ml of dichloromethane, add 73g of aluminum trichloride in four times, and keep the system temperature at -5°C during the addition process. After the addition, react at room temperature for 0.75h, and then react in a water bath at 60°C for 3h. Afterwards, the mixed reactant was slowly dispersed in 300ml of 5% hydrochloric acid ice-water mixed solution. During the pouring process, vigorous stirring was required. After standing still, suction filtration, the filter cake was washed with anhydrous acetone, dried, and o-xylene was recrystallized to obtain white 24g of flaky 2,3,6,7-tetramethylanthracene solid, with a melting point of 299°C.
[0101] 1 HNMR (400MHz, CDCl3): δ=2.43(s,12H),7.68(s,4H),8.13(s,2H)
[0102] Weigh 2g of 2,3,6,7-tetr...
Embodiment 3
[0122] Example 3: Preparation of high-purity 14,15-dimethoxy-2,3,6,7-triptycene tetraacid dianhydride (TM4)
[0123] Under the conditions of ice-salt bath and vigorous stirring, in a 250ml three-neck flask with 120ml of o-xylene and 70ml of dichloromethane, add 50g of aluminum trichloride in four times, and keep the system temperature at 5°C during the addition process. After the addition, react at room temperature for 1.0 h, and then react in a water bath at 70° C. for 5 h. Afterwards, the mixed reactant was slowly dispersed in 300ml of 5% hydrochloric acid ice-water mixed solution. During the pouring process, vigorous stirring was required. After standing still, suction filtration, the filter cake was washed with anhydrous acetone, dried, and o-xylene was recrystallized to obtain white 19g of flaky 2,3,6,7-tetramethylanthracene solid, melting point is 299°C.
[0124] 1 HNMR (400MHz, CDCl3): δ=2.43(s,12H),7.68(s,4H),8.13(s,2H)
[0125] Weigh 2g of 2,3,6,7-tetramethylanthra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com