Plating solution for electroless plating of Ni-W-Zn-P alloy coating and coating process thereof
An alloy coating and electroless plating technology, applied in metal material coating process, liquid chemical plating, coating and other directions, can solve the problem of low alloying degree, limited variation range of coating composition, poor compactness of Ni-W-P alloy coating, etc. problem, to achieve the effect of strong corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
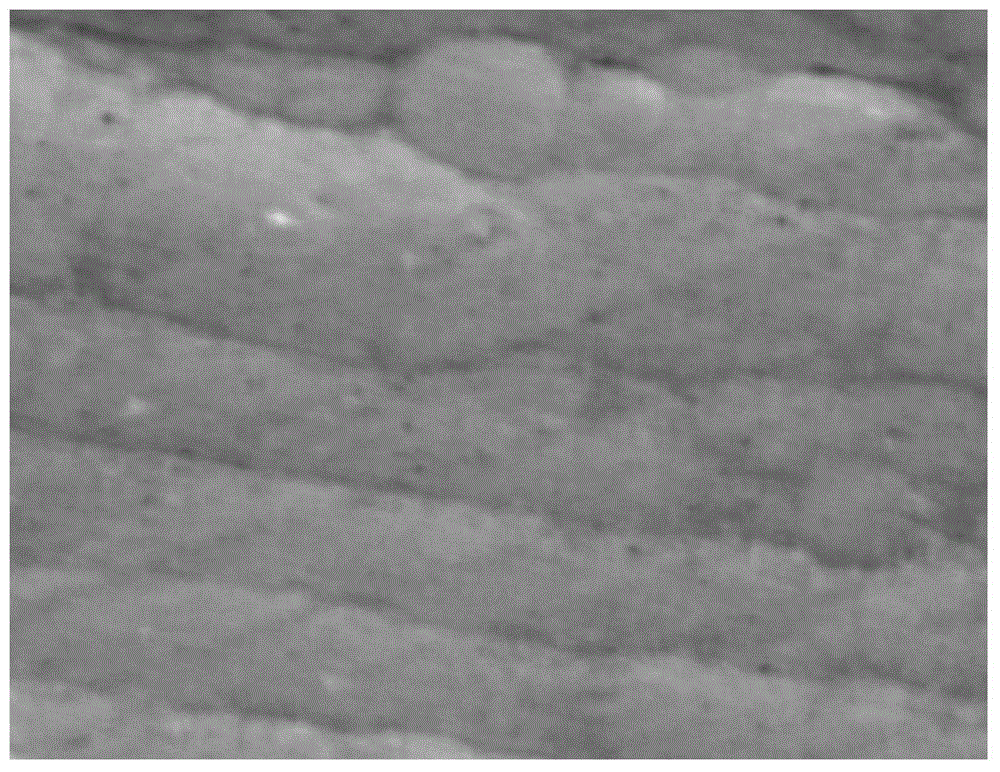
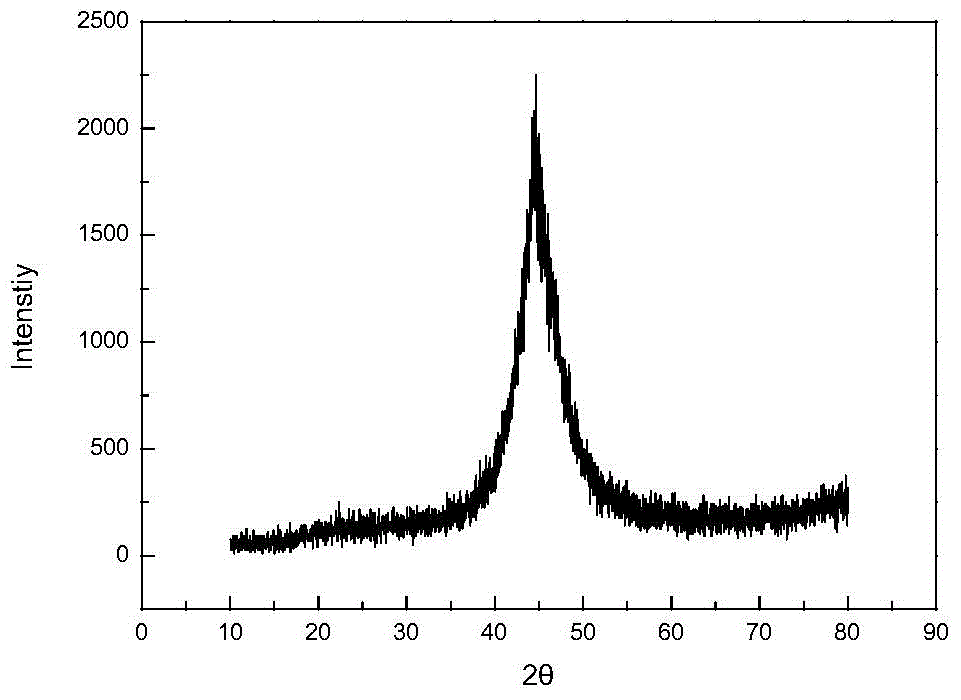
Image
Examples
Embodiment 1
[0082] Step 1: Substrate pretreatment
[0083] A mechanical grinding. Grind the Q235 steel samples with a size of 15mm*15mm*1.5mm with 200#, 400#, 600#, 800#, 1000# and 1500# sandpaper until the surface is basically smooth, and then rinse with water.
[0084] B Degreasing. Soak the sample treated in step A in step 1 with 6% NaOH solution at about 65°C to remove the oil stain on the surface of the sample. The presence of oil stain will seriously affect the bonding force between the coating and the sample. The test method of whether the oil is completely removed is to drop a drop of water on the sample. If the water drop can completely spread the sample, the oil is completely removed. Otherwise, continue to soak to remove oil. Rinse with clean water after degreasing.
[0085] C chemical rust removal. Soak the sample treated in step B in step 1 in 20% sulfuric acid solution for 5-10 minutes to remove surface oxides. Rinse the sample with clean water after derusting.
[0086...
Embodiment 2
[0112] Step 1: Substrate pretreatment
[0113] A mechanical grinding. Grind the Q235 steel samples with a size of 15mm*15mm*1.5mm with 200#, 400#, 600#, 800#, 1000# and 1500# sandpaper until the surface is basically smooth, and then rinse with water.
[0114] B Degreasing. Soak the sample treated in step A in step 1 with 6% NaOH solution at about 65°C to remove the oil stain on the surface of the sample. The presence of oil stain will seriously affect the bonding force between the coating and the sample. The test method of whether the oil is completely removed is to drop a drop of water on the sample. If the water drop can completely spread the sample, the oil is completely removed. Otherwise, continue to soak to remove oil. Rinse with clean water after degreasing.
[0115] C chemical rust removal. Soak the sample treated in step B in step 1 in 20% sulfuric acid solution for 5-10 minutes to remove surface oxides. Rinse the sample with clean water after derusting.
[0116...
Embodiment 3
[0142] Step 1: Substrate pretreatment
[0143] A mechanical grinding. Grind the Q235 steel samples with a size of 15mm*15mm*1.5mm with 200#, 400#, 600#, 800#, 1000# and 1500# sandpaper until the surface is basically smooth, and then rinse with water.
[0144] B Degreasing. Soak the sample treated in step A in step 1 with 6% NaOH solution at about 65°C to remove the oil stain on the surface of the sample. The presence of oil stain will seriously affect the bonding force between the coating and the sample. The test method of whether the oil is completely removed is to drop a drop of water on the sample. If the water drop can completely spread the sample, the oil is completely removed. Otherwise, continue to soak to remove oil. Rinse with clean water after degreasing.
[0145] C chemical rust removal. Soak the sample treated in step B in step 1 in 20% sulfuric acid solution for 5-10 minutes to remove surface oxides. Rinse the sample with clean water after derusting.
[0146...
PUM
| Property | Measurement | Unit |
|---|---|---|
| microhardness | aaaaa | aaaaa |
| microhardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com