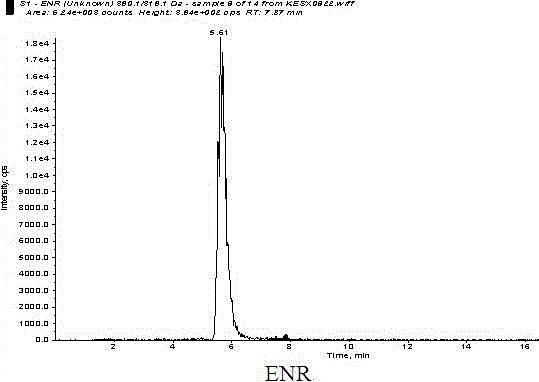
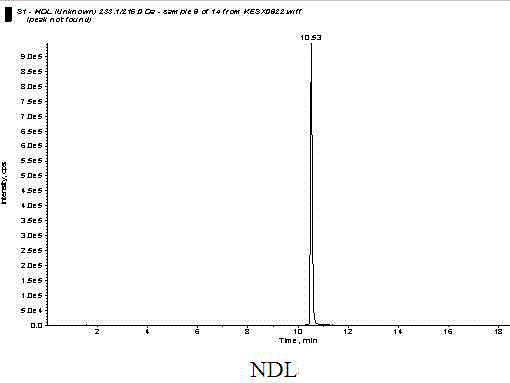
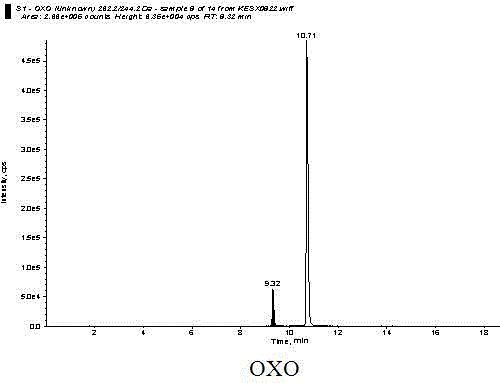
Detection method of quinolones residue in animal tissue
A quinolone and detection method technology, which is applied in the field of detection of quinolone drug residues in animal tissues, can solve the problems of rapid detection of unfavorable samples, long time consumption, etc., achieves good recovery rate and reduces the effect of matrix effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] 1. Sample pretreatment
[0014] Accurately weigh 5.00g of crushed sample, add 20mL of 1% acetic acid to acidify acetonitrile, mix well, ultrasonicate for 20min, add 5g of baked anhydrous sodium sulfate and mix well, centrifuge at 8000r / min for 5min, take about 12mL supernatant Slowly add to the ultrafiltration tube along the tube wall, centrifuge at 5000r / min for 10min, take out 10mL of the centrifuged liquid and dry it with nitrogen gas, and dilute the mobile phase to 1.0mL for HPLC-MS / MS analysis.
[0015] 2. Liquid chromatography conditions
[0016] The chromatographic column used in this example is WatersXbridgeC18, 3.5μm2.1×150mm, Agilent ZorbaxSB-C18, XDB-C18 and other octadecyl bonded phase silica gel can also meet the separation requirements.
[0017] Injection volume: 20 μL; mobile phase: A: 0.1% formic acid solution; B: acetonitrile; flow rate: 0.2 mL / min; see Table 1 for gradient elution conditions.
[0018] Table 1 Liquid Chromatography Gradient Elution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com