Lithium sulfur battery based on modification of graphene oxide thin film
A graphene film, lithium-sulfur battery technology, applied in lithium batteries, battery electrodes, battery pack components, etc., can solve problems such as capacity decline, low energy efficiency, and loss of active material sulfur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 is used for the preparation of the modified sulfur electrode
[0028] The carbon-sulfur composite, binder, and conductive agent were mixed according to a certain ratio and ball-milled for 30 minutes to prepare a sulfur electrode for modification.
[0029] Among them, the binder is polyvinylidene fluoride, and the ratio in the modified sulfur electrode is 5%; the conductive agent is oxidized carbon black, and the ratio in the modified sulfur electrode is 10%.
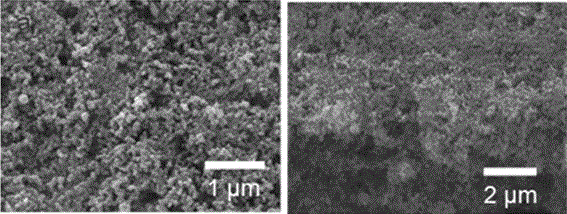
[0030] The morphology of the modified electrode sheet prepared by the above method was characterized, and the SEM image is as follows figure 1 As shown, (a) is the surface topography, (b) is the topography of the cross section; according to the topography (a), it can be seen that the active material is evenly distributed on the current collector aluminum foil, according to the topography (b) It can be seen that the cross-section of the active material is a porous fluffy structure.
Embodiment 2
[0031] Preparation of the sulfur electrode modified by embodiment 2 graphene oxide film
[0032] The carbon-sulfur compound, binder, and conductive agent were mixed according to a certain ratio and ball-milled for 30 minutes to prepare a modified sulfur electrode; wherein, the binder was polyvinylidene fluoride, and the ratio in the modified sulfur electrode was 5%; the conductive agent is oxidized carbon black, and the proportion in the modified sulfur electrode is 10%.
[0033] Graphene oxide was prepared and dissolved in ethanol at a concentration of 1 mg / mL.
[0034] Evenly drop 10mL of the prepared graphene oxide ethanol solution on the surface of a 10cm2 sulfur electrode for modification, and dry it at 50°C to 120°C.
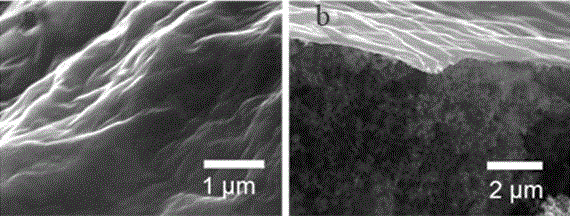
[0035] Morphological characterization of the graphene oxide film-modified sulfur electrode sheet prepared by the above method, the SEM image is as follows figure 2 As shown, (a) is the topography of the surface, and (b) is the topography of the cross ...
Embodiment 3
[0037] Example 3 Lithium-sulfur battery performance test
[0038] The graphene oxide-modified sulfur electrode prepared in Example 2 and the modified sulfur electrode prepared in Example 1 were assembled into a button battery for charge and discharge tests.
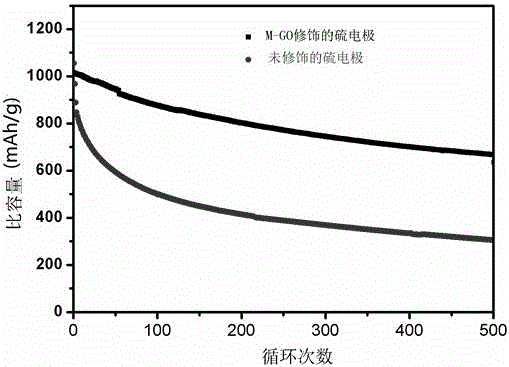
[0039] The test curve of the button battery is as follows image 3 , as shown in 4.
[0040] according to image 3 It can be seen from the cycle number-specific capacity diagram under the test current of 0.5A / g that the initial specific capacity of the unmodified sulfur electrode is about 1050mAh / g, which is slightly higher than the initial specific capacity of the sulfur electrode modified by graphene oxide film 1010mAh / g. g, this may be related to the diffusion rate of lithium ions in the initial state, but it can be seen from the subsequent charge and discharge that the specific capacity of the unmodified sulfur electrode drops sharply. After 100 cycles, the specific capacity is only about 500mAh / g, and the capacit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com