Platinum anticancer oxaliplatin composition
A technology for oxaliplatin and anticancer drugs, which is applied in the field of medicine and can solve the problems of easy moisture absorption and deterioration of loading capacity, poor fluidity, and differences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Oxaliplatin Crystals
[0024] Take the oxaliplatin raw material, add in the mixed solvent A of N,N-dimethylformamide and N-methylacetamide whose volume is 8 times the weight of oxaliplatin at 30°C, N,N-dimethylformamide Methyl formamide, N-methyl acetamide volume ratio is 4:1, obtains solution; Then in the horizontal direction of the liquid surface of gained solution, applying magnetic field intensity is the constant magnetic field of 1.0T, and under the condition of this constant magnetic field to Chloroform whose volume is 8 times the weight of oxaliplatin was added dropwise to the solution; after the dropwise addition, the temperature was lowered to -10°C, allowed to stand for 2 hours, filtered, washed, and vacuum-dried to obtain the oxaliplatin crystals.
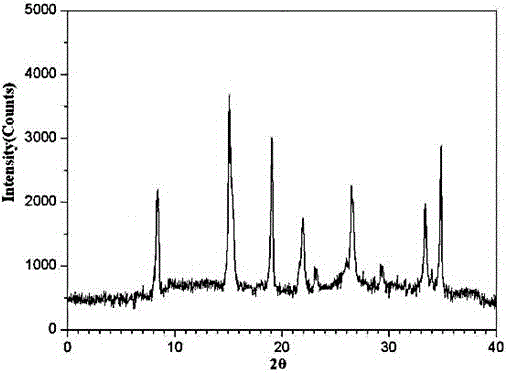
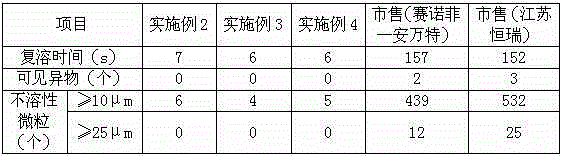
[0025] The X-ray powder diffraction pattern obtained by the Cu-Kα ray measurement of the prepared oxaliplatin crystal is as follows: figure 1 Shown, its purity as determined by high pe...
Embodiment 2
[0026] Example 2: Preparation of Oxaliplatin Compositions
[0027] The composition is: 1 part by weight of oxaliplatin crystal prepared by the present invention, and 0.03 part by weight of sodium dihydrogen phosphate.
[0028] The preparation method is:
[0029] (1) Weigh oxaliplatin crystals and sodium dihydrogen phosphate in proportion and mix them thoroughly;
[0030] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0031] Example 3: Preparation of Oxaliplatin Compositions
[0032] The composition is: 1 part by weight of oxaliplatin crystal prepared by the present invention, and 0.04 part by weight of sodium dihydrogen phosphate.
[0033] The preparation method is:
[0034] (1) Weigh oxaliplatin crystals and sodium dihydrogen phosphate in proportion and mix them thoroughly;
[0035] (2) Dispense into sterilized vials and stopper them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com