Method for preparing carboxymethyl konjac glucomannan nano drug carrying microspheres
A technology of konjac glucomannan and nano-drug-carrying, which can be applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of slow swelling rate of konjac glucomannan and stable sol. problems such as poor performance, to achieve the effect of short cycle, high encapsulation efficiency and protection activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of carboxymethyl konjac glucomannan:
[0032] (1) Accurately measure 70mL of concentrated hydrochloric acid, and dilute to 250mL with absolute ethanol; then accurately weigh 35g of konjac glucomannan and put it into a three-neck flask, add 250mL of prepared ethanol hydrochloric acid solution, and mechanically Stirring and reacting for 2 hours; after the reaction, the product was washed with 70 wt% ethanol aqueous solution to neutrality, filtered under reduced pressure, and vacuum-dried at 30° C. for 16 hours to obtain acid-hydrolyzed konjac glucomannan. It is 1.68mpa.s (and the absolute viscosity of the konjac glucomannan before acidolysis is 82.1mpa.s, measuring solution is 1wt% with NDJ-79 rotary viscometer to measure the konjac glucomannan absolute viscosity after the acid hydrolysis aqueous solution).
[0033] (2) Preparation of carboxymethyl konjac glucomannan: first accurately weigh 10 g of acid-hydrolyzed konjac glucomannan and put it into a three...
Embodiment 2
[0044] Preparation of ovalbumin-carboxymethyl konjac glucomannan nanoparticle drug-loaded microspheres
[0045] The preparation method of carboxymethyl konjac glucomannan and 2-hydroxypropyltrimethylammonium chloride chitosan is the same as in Example 1.
[0046] 1) 4ml concentration is that the ovalbumin aqueous solution of 2mg / mL slowly joins in the 2-hydroxypropyltrimethylammonium chloride chitosan aqueous solution that 10ml concentration is 1.5mg / mL, stirs and regulates the pH of mixed solution to neutral;
[0047] 2) Slowly add 5 ml of carboxymethyl konjac glucomannan aqueous solution with a concentration of 2 mg / mL dropwise to the mixed solution in step 1) while stirring, and continue to stir and react for 15 minutes;
[0048] 3) centrifuging the reaction solution, washing the separated precipitate with water and freeze-drying to obtain ovalbumin-carboxymethyl konjac glucomannan nano drug-loaded microspheres.
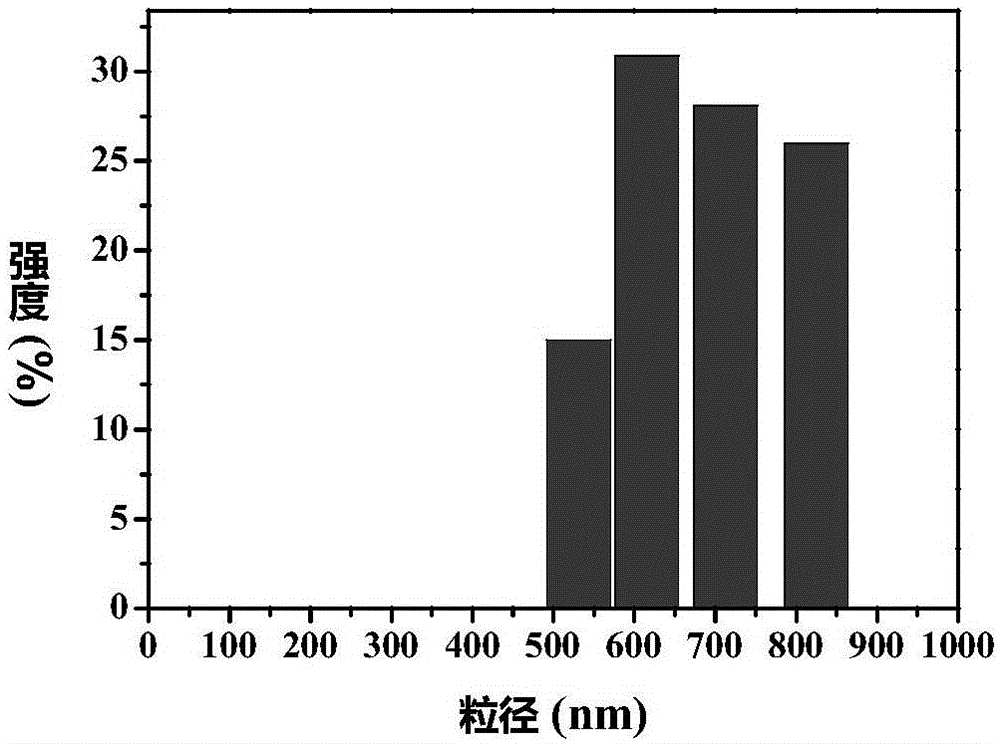
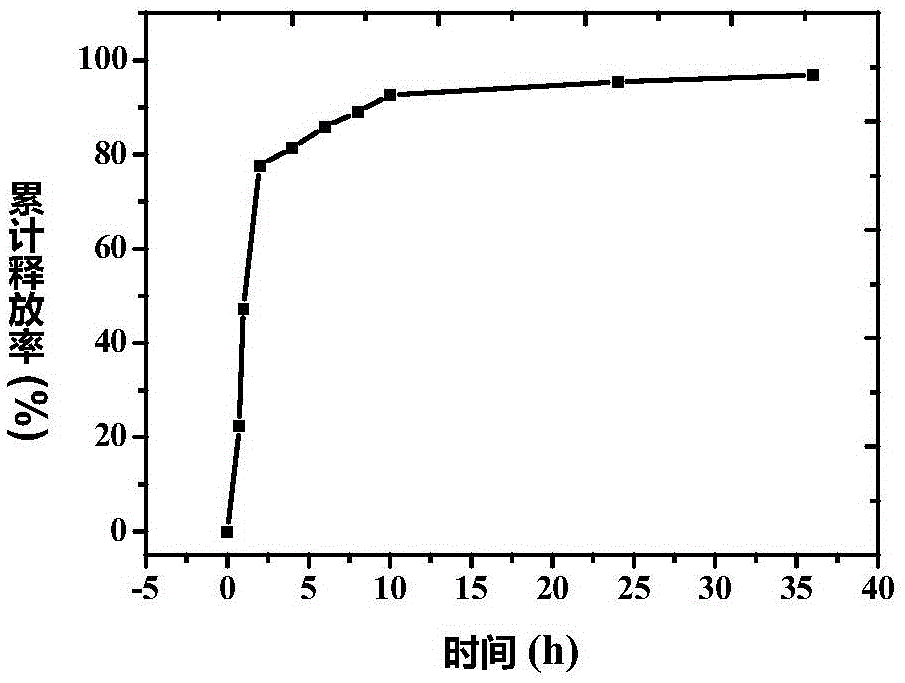
[0049] The particle size distribution and zeta potential o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com